CBSE Class 11-science Answered
The decomposition of a certain mass of CaCO3 gave 11.2 dm3 of CO2 gas at STP . The mass of KOH required to completely neutralise the gas is
a) 56g
b) 28g
c) 42g
d)20g
Asked by Balbir | 21 Jun, 2019, 09:57: PM
Given:
Volume of CO2 = 11.2 dm3
We have,
1 mole of CO2 = 22.4 dm3
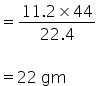
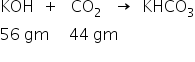
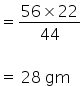
44 gm of of CO2 = 22.4 dm3
x gm of CO2 = 11.2 dm3
x =
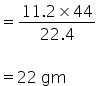
The neutralisation reaction is as,
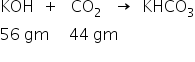
56 gm KOH required for neutralisation of 44 gm of of CO2
KOH required for neutralisation of 22 gm of of CO2
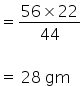
28 gm of KOH is required for complete neutralisation of 22 gm of of CO2.
Answered by Varsha | 23 Jun, 2019, 10:32: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM







