CBSE Class 11-science Answered
State two basic characteristics of the ideal gas. Are real gases perfect gases?
Asked by Topperlearning User | 07 May, 2015, 11:44: AM
The two basic characteristics of the ideal gas are:
(i) The size of the molecule of a gas is zero.
(ii) The molecules of a gas do not exert any force of attraction or repulsion amongst each other.
All real gases are not perfect gases, as they do not obey gas laws perfectly.
Answered by | 07 May, 2015, 13:44: PM
Concept Videos
CBSE 11-science - Physics
Asked by pratikshyadashrkl | 01 May, 2020, 10:24: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 07 May, 2015, 11:44: AM
CBSE 11-science - Physics
(a) In equation, PV = RT, what does V stand for?
(b) In the equation, P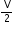 = RT, what does V stand for?
= RT, what does V stand for?
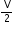 = RT, what does V stand for?
= RT, what does V stand for?
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 07 May, 2015, 11:46: AM
CBSE 11-science - Physics
Asked by thakursonali2000 | 14 Dec, 2015, 17:51: PM



