CBSE Class 11-science Answered
Dear experts,
Kindly explain this question with a proper solution.
Thank you.

Asked by pratikshyadashrkl | 01 May, 2020, 10:24: AM
We know,
Ideal gas equation is,
PV = nRT ....(1)
From given information we can see that pressure (P) of gas remains unaffected becasue there is no change in n, R, T and V.
Inside the vessel, the molecules of gas undergoes perfect elastic collison and hence, number of collision of gas molecules per unit volume remians constant and thus, the pressure inside the vessel remains constant.
Hence, the pressure of the gas inside the vessel as observed by us on the ground remains same because motion of vessel as a whole does not affect the relative motion of molcules of gas and walls.
Option - (b)
Answered by Shiwani Sawant | 01 May, 2020, 12:17: PM
Concept Videos
CBSE 11-science - Physics
Asked by pratikshyadashrkl | 01 May, 2020, 10:24: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 07 May, 2015, 11:44: AM
CBSE 11-science - Physics
(a) In equation, PV = RT, what does V stand for?
(b) In the equation, P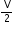 = RT, what does V stand for?
= RT, what does V stand for?
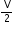 = RT, what does V stand for?
= RT, what does V stand for?
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 07 May, 2015, 11:46: AM
CBSE 11-science - Physics
Asked by thakursonali2000 | 14 Dec, 2015, 17:51: PM



