CBSE Class 11-science - Behaviour of Ideal Gases Videos
Kinetic Theory of Gases
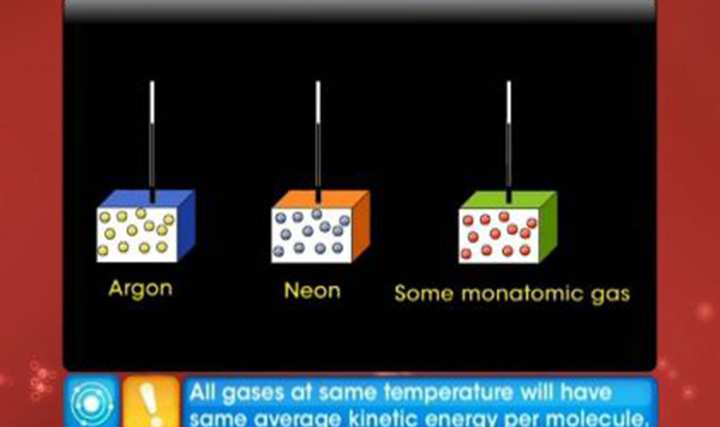
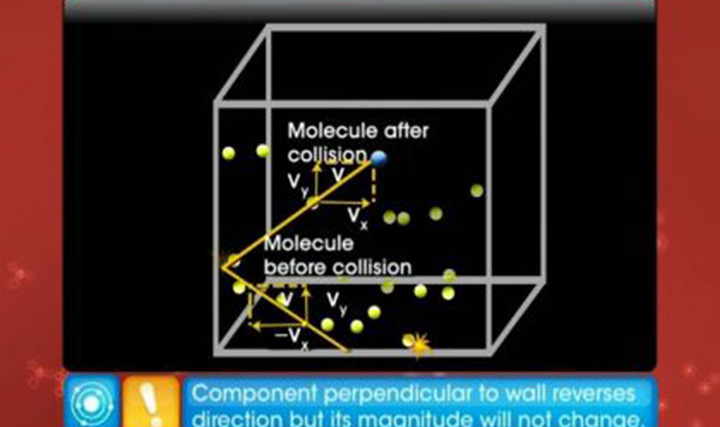
This video explains the kinetic theory of gases, ideal gas law and behaviour of an ideal gas.
More videos from this chapter
View All-
Dear experts,
Kindly explain this question with a proper solution.
Thank you.

- What do you mean by an ideal gas?
- State two basic characteristics of the ideal gas. Are real gases perfect gases?
-
(a) In equation, PV = RT, what does V stand for?
(b) In the equation, P
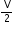 = RT, what does V stand for?
= RT, what does V stand for?
- When pressure increases by 1%, what is the percentage decrease in the volume of a gas, if Boyle's law is obeyed.
- Find the number of molecules in one cubic meter of an ideal gas at N.T.P
- During an experiment, an ideal gas is found to obey an additional law VP2 = constant. The gas is initially at a temperature T and volume V. When it expands to a volume 2V, the temperature becomes..................
- An electric bulb of volume 250 cm3 was sealed off during manufacture at the pressure of 10-3 mm of mercury at 27 oC. Find the number of air molecules in the bulb.
- <div>Find time(in years)to spend 1 mole of rupees ,if we spend 100 crore rupees in one day.</div>