CBSE Class 11-science Answered
An electric bulb of volume 250 cm3 was sealed off during manufacture at the pressure of 10-3 mm of mercury at 27 oC. Find the number of air molecules in the bulb.
Asked by Topperlearning User | 07 May, 2015, 11:46: AM
Let n be the number of air molecules in the bulb.
Given : V1 = 250 cm3, P1 = 10-3 mm of Hg, T1 = 300 K
Using PV = nRT, where R is the universal gas constant, we get
10-3 x 250 = nR x 300 .............(i)
At N.T.P, one molecule of air occupies a volume of 22400 cm3. So, at N.T.P.,
V2 = 22400 cm3, P2 = 760 mm of Hg,
T2 = 273 K, n2 = N = 6 x 1023 molecules
Therefore, 760 x 22400 = 6 x 1023 x R x 273 ...........(ii)
Dividing (i) by (ii), we get
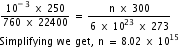
Answered by | 07 May, 2015, 13:46: PM
Concept Videos
CBSE 11-science - Physics
Asked by pratikshyadashrkl | 01 May, 2020, 10:24: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 07 May, 2015, 11:44: AM
CBSE 11-science - Physics
(a) In equation, PV = RT, what does V stand for?
(b) In the equation, P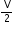 = RT, what does V stand for?
= RT, what does V stand for?
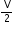 = RT, what does V stand for?
= RT, what does V stand for?
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 07 May, 2015, 11:46: AM
CBSE 11-science - Physics
Asked by thakursonali2000 | 14 Dec, 2015, 17:51: PM



