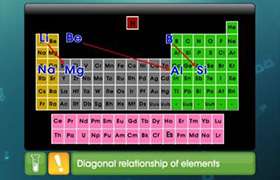
CBSE Class 11-science Answered

As a general rule, electron gain enthalpy becomes more negative with increase in atomic number across a period. The atomic size decreases and effective nuclear charge increases as we go from left to right across a period and consequently it will be easier to add an electron to smaller atom since the added electron on an average would be closer to the positively charged nucleus.
The trends in electron gain enthalpy values within a period are irregular for elements of group 2, group 15 and group 18 since they have atoms with symmetrical configuration (having filled and half-filled orbitals in the same sub-shell) and hence do not have any urge to take up extra electrons because their configuration will become unsymmetrical or less stable.