CBSE Class 12-science Answered
sir/madam,
this doubt will be lenghty. request peace reading. the doubt is w.r.t chapter Amines; to be specific it is w.r.t basicity of amines.
in the topic of the comparision of the basic character of alkylamines with ammonia,
the order of basicity of amines is tertiary> secondary> primary in gaseous phase, only due to the +I effect.
in gasous phase only +I effect is shown?
also in the aqueous phase, the disturbancy in the order of basicity is due to the sum of effects of +I effect, solvation effect and steric hindrance?
the order of the stability of the following in aqueous phase:
+I effect: tertiary> secondary> primary
solvation: primary > secondary > tertiary
steric hindrance: primary > secondary > tertiary
so does it means that +I is effected in both gaseous and aqueous phase but solvation and steric hindrance are effected only in aqueous phase?
also sir there is an order of basicity when R= CH3 group and when R= C2H5 group:
R= CH3:
secondary> primary> tertiary
R= C2H5:
secondary> tertiary> primary
so sir can we generalise the order that:
when R=CH3 group, the order of basicity:
secondary> primary> tertiary
and when R> CH3 group, the order of basicity:
secondary> tertiary> primary
Asked by kaziryan.05 | 12 Jul, 2021, 21:51: PM
Dear Student,
We're glad that you asked such conceptual question.
Let's understand the concept one by one and than you can answer all the type of questions.
Firstly, Let's talk about gaseous state,
In gaseous effect two major factos affect the basicity of amines.
(1) +I effect
(2) Steric Hinderance
Solvation effect is due to H bonding with water in aqueous solution. In gaseous solution, there is no water solvent so solvation effect doesn't occur.
Steric hinderance is less effetive becausee CH3 is smaller in sizee. If It was C2H5 than definetly we would consider it.
So Order of basicity is primarily due to +I effect and it will be-
Teriary>Secondary>Primary (For RCH3)
Now, We can take about aqueous solution,
3 factors work on it-
1. +I Efffect
2. Solvation
3. Steric Hinderance.
Steric Hinderance can't be ignored because in aqueous solution intermolecular space is laready less.
Solvation effeect comes on priority because it forms H bond with water and I effect will be less effective.
Ordrer should be Primary>secondary>Tertiary
But Steric Hinderance is effective in Tertiaory amine so order will effect only that. Secondary> Primary because of I effect.
So, Order is-
Secondary>Primary>Teritary (For R-CH3)
Answered by Ravi | 15 Jul, 2021, 12:01: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 06 Jul, 2021, 23:31: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 13:35: PM
CBSE 12-science - Chemistry
Asked by Sudamkalgunde624 | 31 Dec, 2019, 11:38: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 19 Nov, 2019, 12:39: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 19 Jun, 2019, 21:19: PM
CBSE 12-science - Chemistry
Asked by afiaorpi01 | 22 Mar, 2019, 01:19: AM
CBSE 12-science - Chemistry
Asked by abhitailor158 | 07 Mar, 2019, 16:44: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 14:44: PM


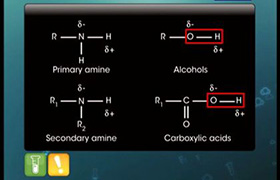

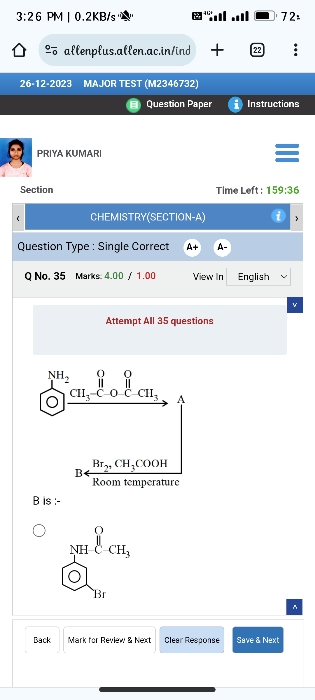
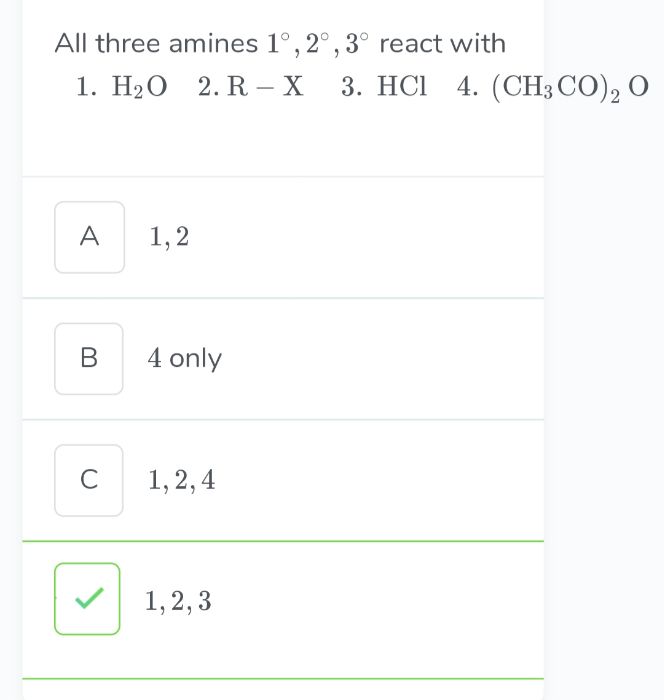
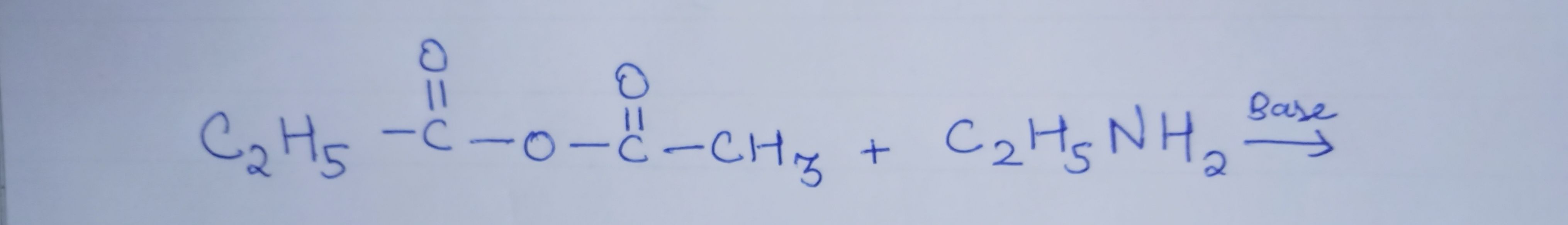

 Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2
Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2