CBSE Class 12-science Answered

(ii) Decreasing order of basic strength:
p- Toluidine > aniline > p- nitroaniline
This is because -NO2 group has an electron withdrawing inductive effect or -I effect and -CH3 group has electron releasing inductive effect or +I effect. Groups with -I effect decreases the electron density on the nitrogen of amino group and hence decreases the basic strength. Groups with +I effect increases the electron density on the nitrogen of amino group and hence increases the basic strength.
(iii) Increasing order of pKb value:
(C2H5)2NH < C2H5NH2 < C6H5NHCH3 < C6H5NH2
This is because -C6H5 group has an electron withdrawing inductive effect or -I effect and -C2H5 group has electron releasing inductive effect. Groups with -I effect decreases the electron density on the nitrogen of amino group and hence decreases the basic strength. Groups with +I effect increases the electron density on the nitrogen of amino group and hence increases the basic strength. Greater the basic strength the smaller is the pKb value.


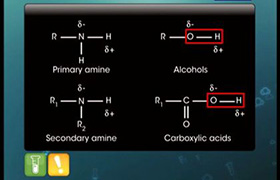

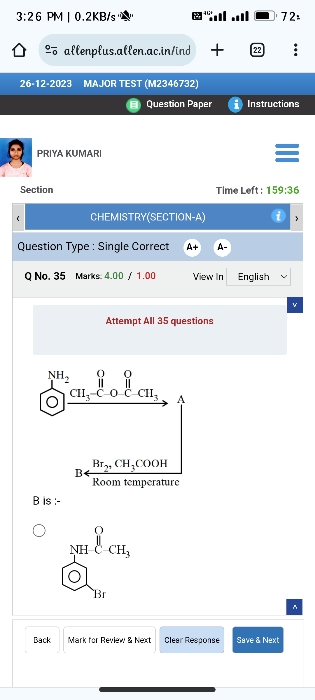
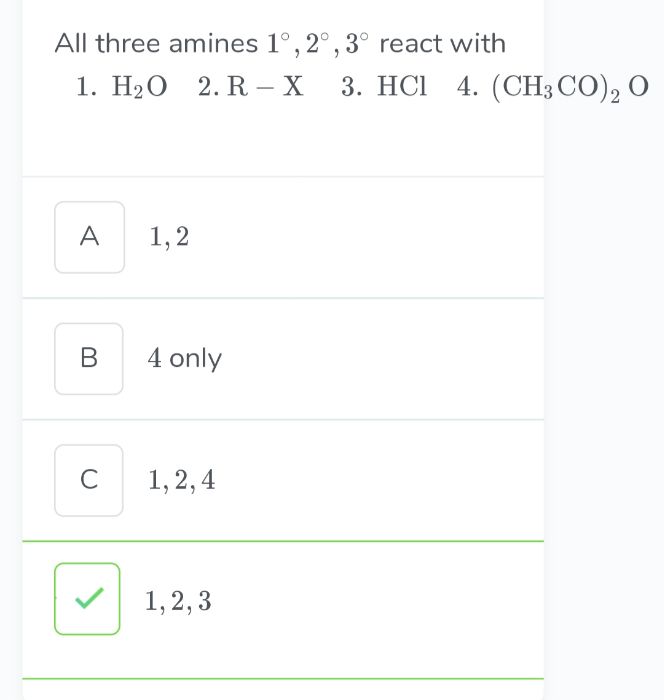
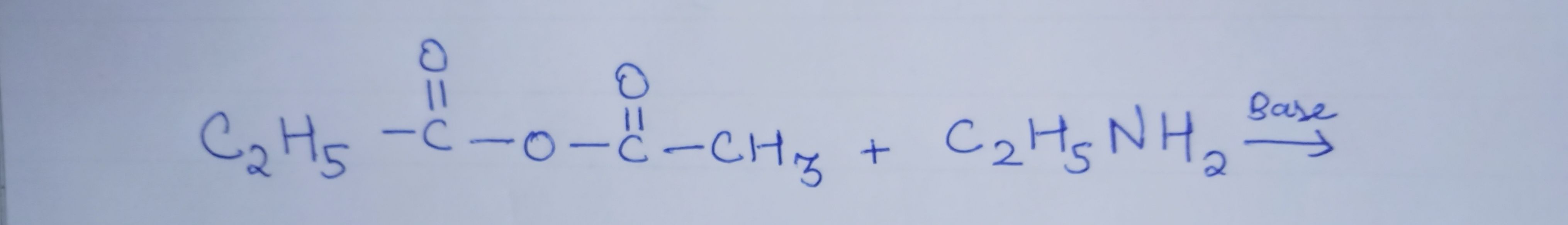
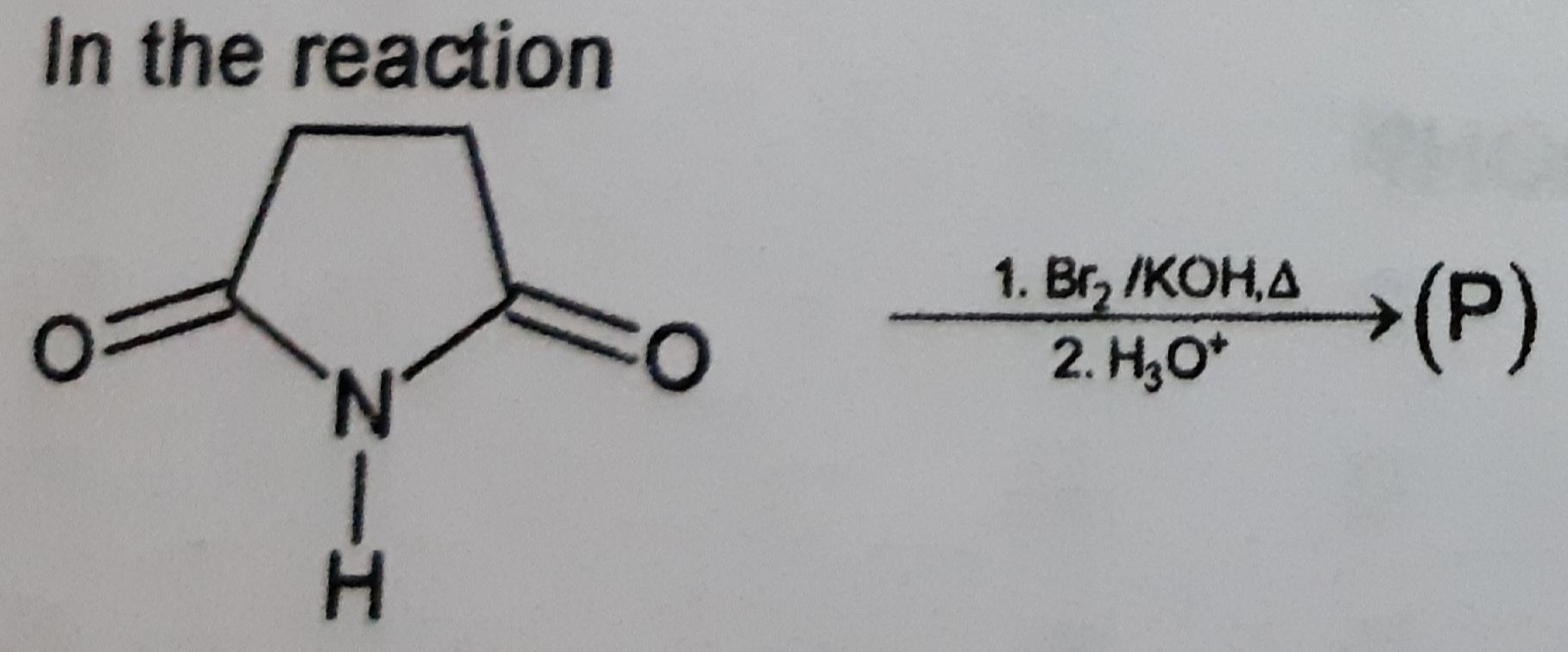
 Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2
Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2