CBSE Class 12-science - Properties of Amines Videos
Properties of Amines
Physical and chemical properties of amines
More videos from this chapter
View All-
ans me
- sir/madam, this doubt will be lenghty. request peace reading. the doubt is w.r.t chapter Amines; to be specific it is w.r.t basicity of amines. in the topic of the comparision of the basic character of alkylamines with ammonia, the order of basicity of amines is tertiary> secondary> primary in gaseous phase, only due to the +I effect. in gasous phase only +I effect is shown? also in the aqueous phase, the disturbancy in the order of basicity is due to the sum of effects of +I effect, solvation effect and steric hindrance? the order of the stability of the following in aqueous phase: +I effect: tertiary> secondary> primary solvation: primary > secondary > tertiary steric hindrance: primary > secondary > tertiary so does it means that +I is effected in both gaseous and aqueous phase but solvation and steric hindrance are effected only in aqueous phase? also sir there is an order of basicity when R= CH3 group and when R= C2H5 group: R= CH3: secondary> primary> tertiary R= C2H5: secondary> tertiary> primary so sir can we generalise the order that: when R=CH3 group, the order of basicity: secondary> primary> tertiary and when R> CH3 group, the order of basicity: secondary> tertiary> primary
- sir/ madam, is it true that if no of bonds in an atom increases then the atom acquires a positive charge on it and when it loses one bond it acquires a negative charge on it ? why does it happen so?
-
pls explain
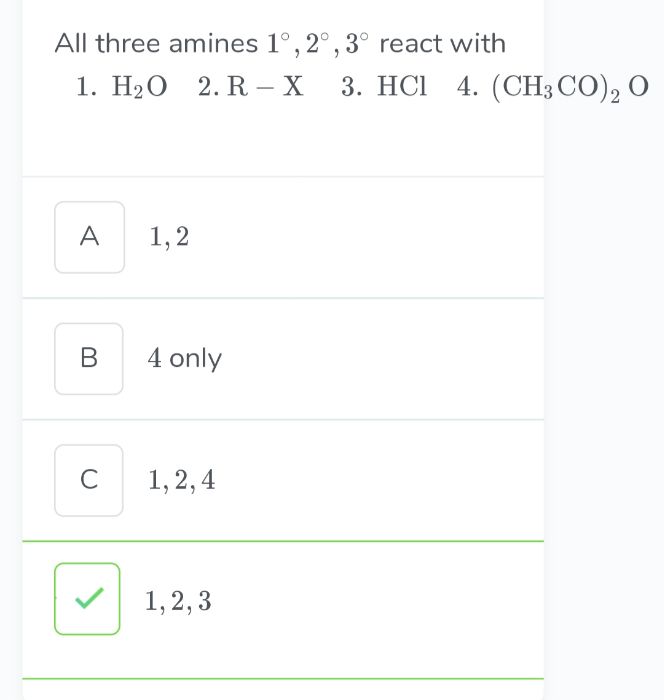
- Conversion of amines
-
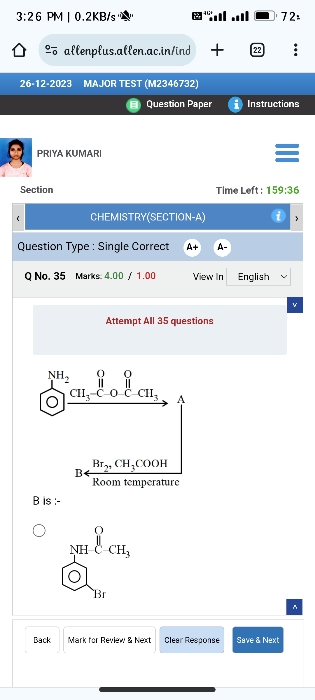
What is the product of this amide acylation?
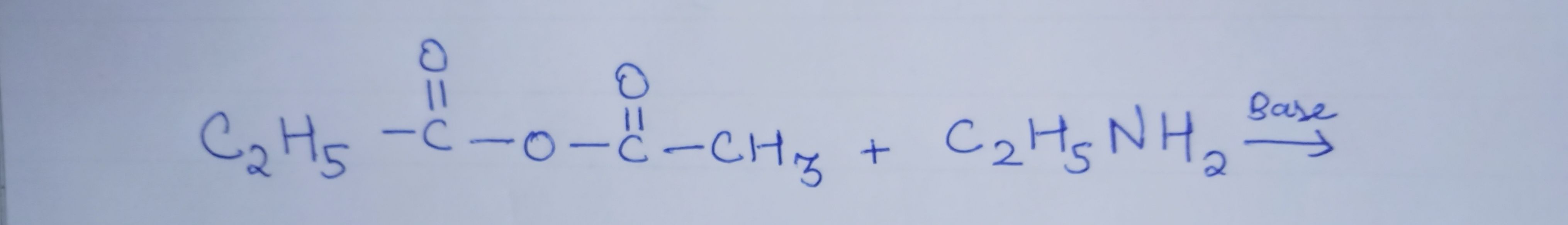
-
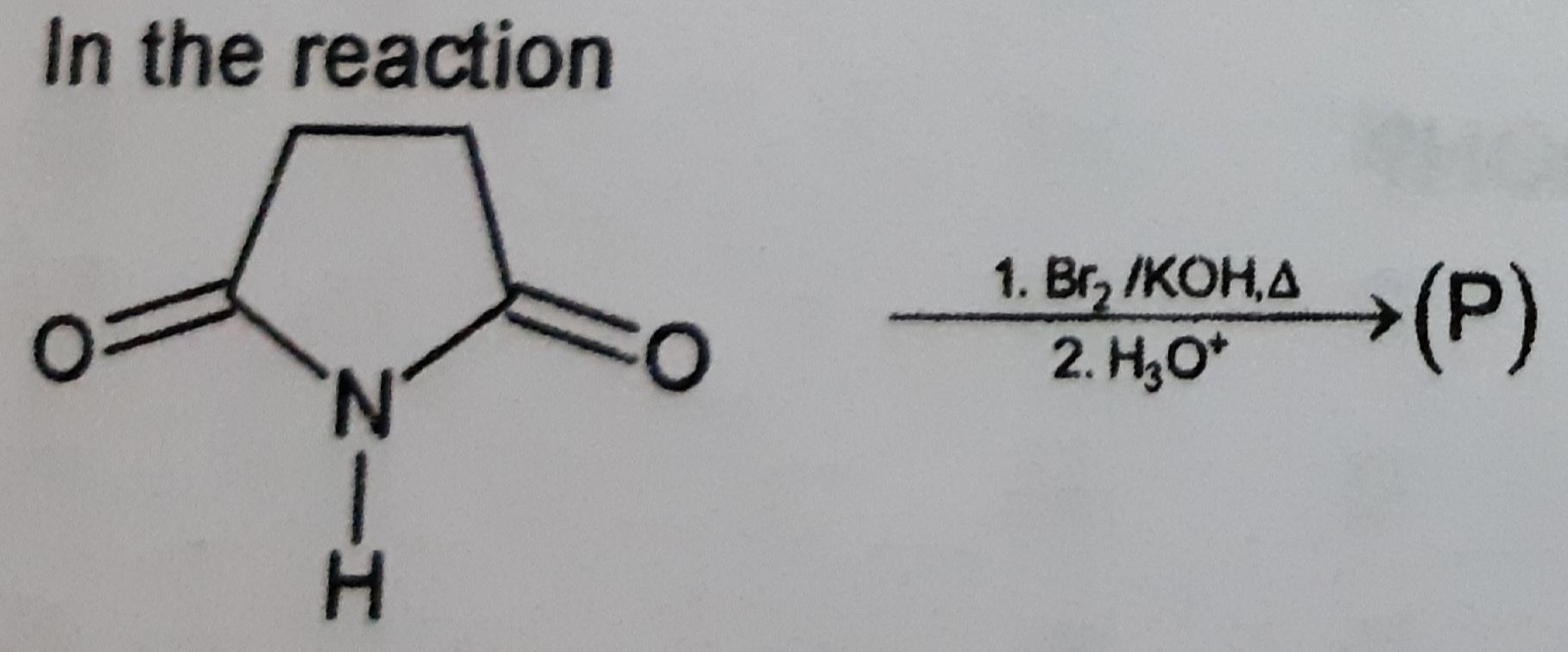
Please answer this question.
- Why toluene is more reactive than nitro benzene?
-
Question 6. Option 2nd and 3rd

-
 Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2
Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2






