CBSE Class 11-science Answered
Prove that if uncertainty in position of a moving electron is equal to its de-Broglie wavelength than its velocity is completely uncertain?
Asked by fizajain21 | 18 Dec, 2020, 12:46: PM
According to heisenberg's uncertanity-
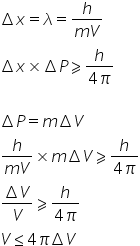
You can see Velcoity is uncertain.
Answered by Ravi | 22 Dec, 2020, 19:27: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by dhondesainath | 28 Sep, 2021, 07:45: AM
CBSE 11-science - Chemistry
Asked by jaidawra48 | 13 Aug, 2021, 18:30: PM
CBSE 11-science - Chemistry
Asked by aayushiyadav408 | 12 Jul, 2021, 15:26: PM
CBSE 11-science - Chemistry
Asked by anandkumar10.12.96 | 28 Dec, 2020, 14:34: PM
CBSE 11-science - Chemistry
Asked by fizajain21 | 18 Dec, 2020, 12:46: PM
CBSE 11-science - Chemistry
Asked by darshanabhamare72 | 16 Oct, 2020, 18:31: PM
CBSE 11-science - Chemistry
Asked by brahampreetkaur818 | 15 Oct, 2020, 22:13: PM
CBSE 11-science - Chemistry
Asked by anish.4006 | 18 Sep, 2020, 20:00: PM





