CBSE Class 11-science Answered
Pls solve it.

Asked by pb_ckt | 16 May, 2019, 03:44: PM
The correct option is (2)
Given:
2 moles of each N2 , H2 and NH3 is present.
Initial weight of N2 = ?
Initial weight of H2 =?
The reaction is,
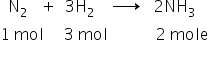
Now, it is given that 2 moles of NH3 is formed.
From reaction it is clear that, to produce 2 moles of NH3 1 mole of N2 and 3 H2 moles of are required.
Also it is geven that 2 moles of N2 and H2 are remained after formation of NH3.
Therefore the initial mole of N2 = 2+1
= 3 mole
We know,
1 mole of N2 = 28 gm
3 moles of of N2 =84 gm
Now
the initial mole of H2= 3+2
= 5 mole
We know,
1 mole of H2 = 2 gm
5 moles of of H2 =10 gm
Therefore the initial weight of N2 and H2 is 84 gm and 10 gm respectively.
Answered by Varsha | 17 May, 2019, 11:16: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreekrishnampatil | 27 Apr, 2024, 10:31: PM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM







