CBSE Class 11-science Answered
pls help me to solve the 59th question

Asked by thejasvi001 | 02 Jan, 2021, 19:49: PM
Fast and sudden expansion of gas is adiabatic process.
For adiabatic expansion of gas, we have,
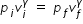 ......................(1)
......................(1)where p is pressure and v is volume . subscript i stands for initial state and subscript f stands for final state.
γ is ratio of specific heat .
we are given that , specific heat at constant volume , Cv = 2R
we have , Cp - Cv = R , where Cp is specific heat at constant pressure.
Hence Cp = 2R+R = 3R and γ = ( Cp / Cv ) = (3R / 2R ) = 1.5
Hence ratio of initial pressure to final pressure is determined from eqn.(1) as
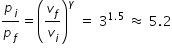
-------------------------
Answer :- Ratio of initial to final pressure is nearly equal to 5
Answered by Thiyagarajan K | 02 Jan, 2021, 21:25: PM
Concept Videos
CBSE 11-science - Physics
Asked by barunmandal12345jmt | 11 Jul, 2024, 16:23: PM
CBSE 11-science - Physics
Asked by chandinichauhai | 10 Jul, 2024, 23:32: PM
CBSE 11-science - Physics
Asked by sulthanxx | 08 Jul, 2024, 19:29: PM
CBSE 11-science - Physics
Asked by bhuvana.s3001 | 21 Jun, 2024, 23:21: PM
CBSE 11-science - Physics
Asked by contact.asmita03 | 15 Jun, 2024, 11:51: AM
CBSE 11-science - Physics
Asked by parthjain2448 | 12 Jun, 2024, 15:53: PM
CBSE 11-science - Physics
Asked by kaivalyaam | 12 Jun, 2024, 03:24: AM
CBSE 11-science - Physics
Asked by tkanmani022 | 11 Jun, 2024, 20:50: PM
CBSE 11-science - Physics
Asked by duraisamysteephen | 08 Jun, 2024, 12:32: PM











