CBSE Class 11-science Answered
Please explain hybridisation in alkanes,alkenes and alkynes.
Asked by Benjamin | 06 Sep, 2015, 05:47: PM
Alkanes have sp3 hybridisation as follows:
- The electronic configuration of carbon in the ground state is 2s22px12py1
- Carbon form four bonds by promoting one of the 2s electrons to 2pz orbital.
- Hence, the electronic configuration of carbon in the excited state is 2s12px12py12pz1.
- These singly occupied four orbitals hybridise to form four sp3 hybrid orbitals.
- They have 109º angle between each of the two sp3 bonds.
- The geometry is tetrahedral.
Hybridisation in alkanes, alkenes and alkynes is as follows:
|
|
Alkyne |
Alkene |
Alkane |
|
Hybridisation of carbon to which H atom is attached |
sp |
sp2 |
sp3 |
|
Percentage s character of carbon |
50% (maximum) |
66.6% |
25% (minimum) |
|
Electronegativity of carbon atom |
Highest |
Less than alkyne and more than alkane |
Lowest |
|
Extent of attraction of hydrogen atoms of C-H bonds towards C |
Highest |
Less than alkyne and more than alkane |
Lowest |
|
Ease of liberation of H atoms as protons |
Highest |
Less than alkyne and more than alkane |
Lowest |
|
Acidic character |
Highest |
Less than alkyne and more than alkane |
Lowest |
Answered by Prachi Sawant | 07 Sep, 2015, 10:14: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


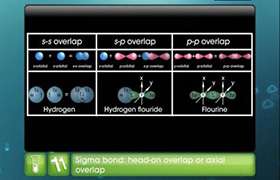
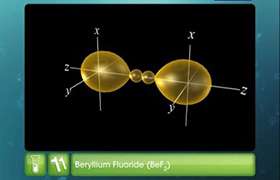
 on the basis of hybridisation
on the basis of hybridisation