CBSE Class 11-science Answered
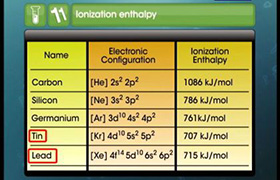
PbO2 is stronger oxidizing agent than SnO2.
Asked by Topperlearning User | 31 May, 2016, 13:25: PM
- In Sn +4 oxidation state is more stable than +2 oxidation state.
- But in case of Pb due to inert pair effect +2 oxidation state is more stable and so Pb+4 gets converted into Pb+2 easily and acts as a good oxidizing agent.
Answered by | 31 May, 2016, 15:25: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by singhnikita8717 | 05 Aug, 2021, 10:44: AM
CBSE 11-science - Chemistry
Asked by jaswindernkd | 21 Jun, 2020, 17:04: PM
CBSE 11-science - Chemistry
Asked by Molaypaul700 | 10 Feb, 2020, 22:32: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 09:44: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:25: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:25: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:14: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:14: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:34: PM