CBSE Class 11-science Answered
Carbon dioxide is non-polar while water is polar. What conclusion do you draw about their structures from this?
Asked by Topperlearning User | 13 Aug, 2014, 09:44: AM
CO2 is linear, bond moments are equal and opposite, so net dipole moment is zero whereas Water is a bent molecule, and has a net dipole moment.
Answered by | 13 Aug, 2014, 11:44: AM
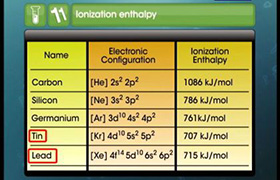
Concept Videos
CBSE 11-science - Chemistry
Asked by singhnikita8717 | 05 Aug, 2021, 10:44: AM
CBSE 11-science - Chemistry
Asked by jaswindernkd | 21 Jun, 2020, 17:04: PM
CBSE 11-science - Chemistry
Asked by Molaypaul700 | 10 Feb, 2020, 22:32: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 09:44: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:25: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:25: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:14: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 13:14: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:34: PM