CBSE Class 11-science Answered
On the basis of kinetic theory explain why, ammonium chloride sublimes and goes from solid state directly into vapour state
Asked by anitabahuguna | 04 May, 2019, 21:57: PM
When ammonium chloride absorbs heat then solid aprticleds filled with energy and this energy is sufficient to break its intermoleucal forces. So intermolecular distance increases and solids are converted directly in gases. This transition from Solid to vapour is rapid. so it can be seen as direct Solid to gas transtion.
Answered by Ravi | 05 May, 2019, 15:00: PM
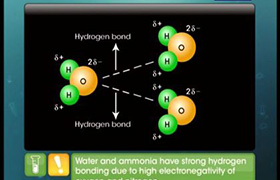
Concept Videos
CBSE 11-science - Chemistry
Asked by neerudhawanbpsmv | 28 Jun, 2021, 09:22: AM
CBSE 11-science - Chemistry
Asked by swatipuspapatel | 10 Jun, 2020, 18:19: PM
CBSE 11-science - Chemistry
Asked by vanyashree106 | 13 Dec, 2019, 21:38: PM
CBSE 11-science - Chemistry
Asked by Asif.k1992 | 12 Sep, 2019, 14:41: PM
CBSE 11-science - Chemistry
Asked by ajitbhot73 | 25 Aug, 2019, 11:52: AM
CBSE 11-science - Chemistry
Asked by design1.bharatifire | 21 Jun, 2019, 18:35: PM
CBSE 11-science - Chemistry
Asked by anitabahuguna | 04 May, 2019, 21:57: PM
CBSE 11-science - Chemistry
Asked by Jatinaa786 | 19 Sep, 2018, 02:53: AM
CBSE 11-science - Chemistry
Asked by arjunshrivastav668 | 17 Mar, 2018, 10:21: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM