CBSE Class 11-science Answered
making use of the concept of hybridisation discuss the shape of:
BeCl2,Bf3,and Ch4 molecules?
Asked by rajenderdagar123 | 11 Jan, 2017, 08:58: PM
Structure of BeCl2:
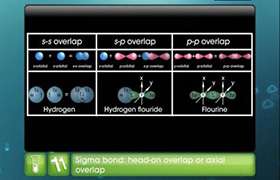
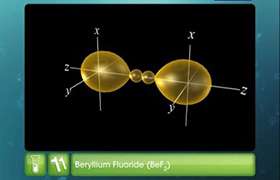
- In BeCl2, the two singly occupied orbitals (2s and 2p) hybridise to give two sp-hybrid orbitals. These hybrid orbitals lie along the z-direction and point in opposite directions.
- The ground state electronic configuration of Be is 1s22s2. In the exited state, one of the 2s-electrons is promoted to the vacant 2p orbital to account for its bivalency.
- One 2s and one 2p-orbital hybridise to form two sp hybridised orbitals. These two sp hybrid orbitals are oriented in opposite directions formi
Answered by Vaibhav Chavan | 12 Jan, 2017, 12:03: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


 on the basis of hybridisation
on the basis of hybridisation