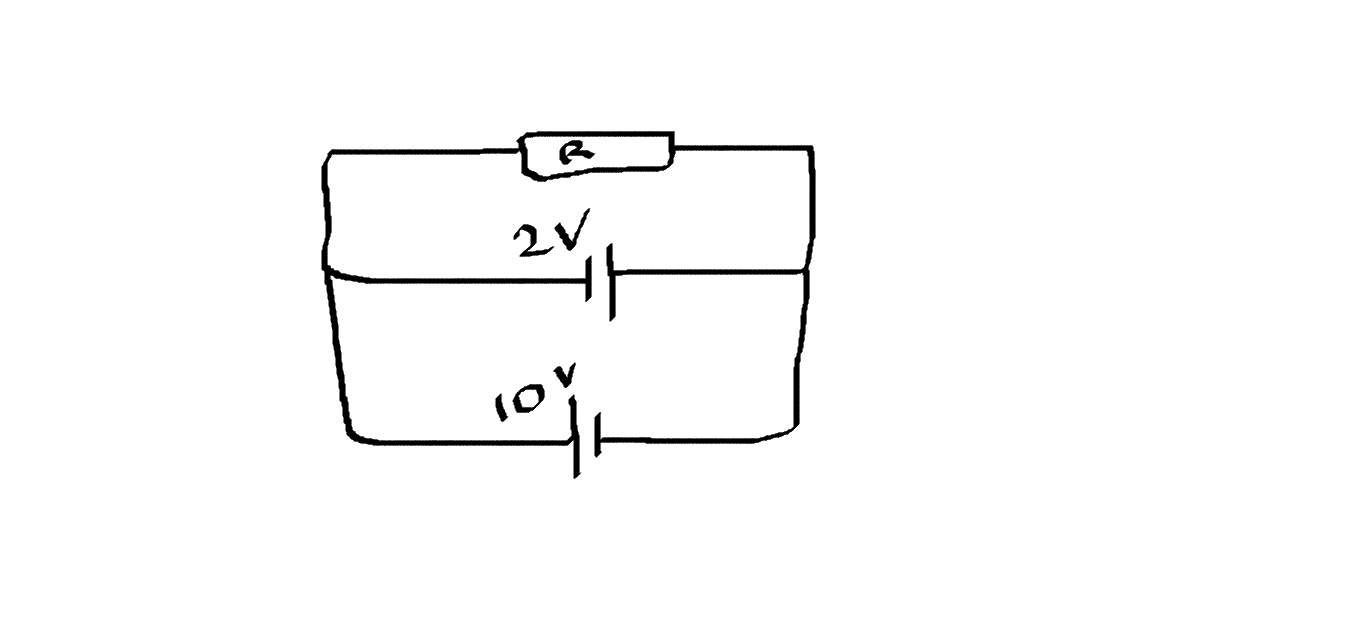
CBSE Class 12-science Answered
in Current electricity,
in section batteries and EMF,
we studied that,in a battery a internal mechanism exerts forces on the charges of battery...
sir can you please explain, how this force drives the charge in a battery and why these forces drive charges in that manner?
please explain sir..
Asked by yashrajgharte24.12dgatl | 18 Aug, 2021, 21:29: PM
In electrical cell chemical energy is converted to electrical energy. In every electrical cell , there are two electrodes
dipped in chemical solutions. One elctrode dipped in chemical solution is isolated from other electrode that is dipped
in a different chemical solution . Chemical reaction is taking place between electrodes and chemical solutions.
In one electode, oxidtation reaction takes place so that electrons released from metal electrode and this electrode
become +ve charge anode . In other electrode, reduction reaction takes place so that electron is absorbed by this elctrode
and it becomes negatively charged cathode.
Due to the presence of charges around electrodes , potential difference is developed and this potential difference is known as
EMF of cell. When these elctrodes are connected externally through a bulb or device that has the ability to draw the current ,
then charges created in chemeical reactions inside the cell will flow through electrodes and either a bulb or device .
Answered by Thiyagarajan K | 18 Aug, 2021, 22:22: PM
Concept Videos
CBSE 12-science - Physics
Asked by guptakartikgupta2 | 16 Jul, 2022, 17:23: PM
CBSE 12-science - Physics
Asked by yashrajgharte24.12dgatl | 18 Aug, 2021, 21:29: PM
CBSE 12-science - Physics
Asked by diyasinha1701 | 31 Jul, 2021, 09:58: AM
CBSE 12-science - Physics
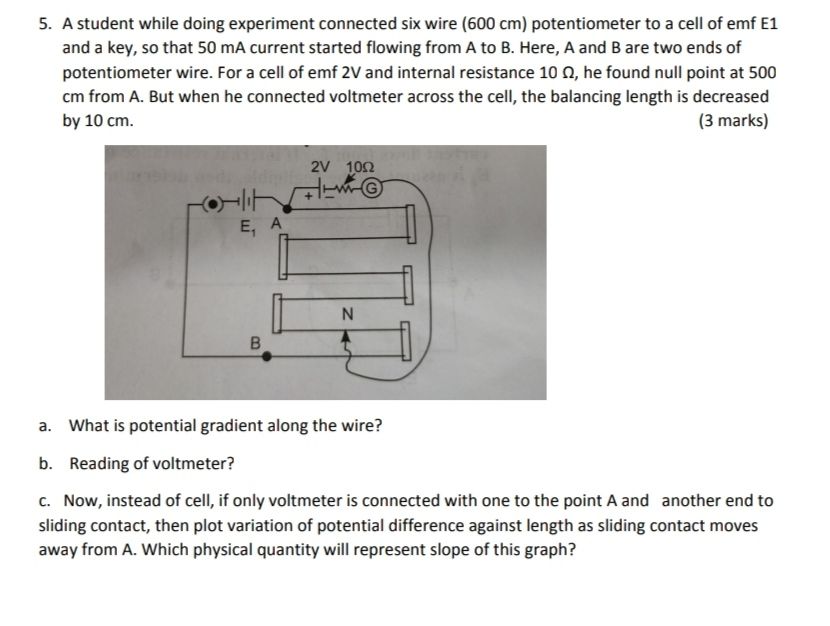
Asked by sulaikhasulu393 | 29 Apr, 2020, 17:17: PM
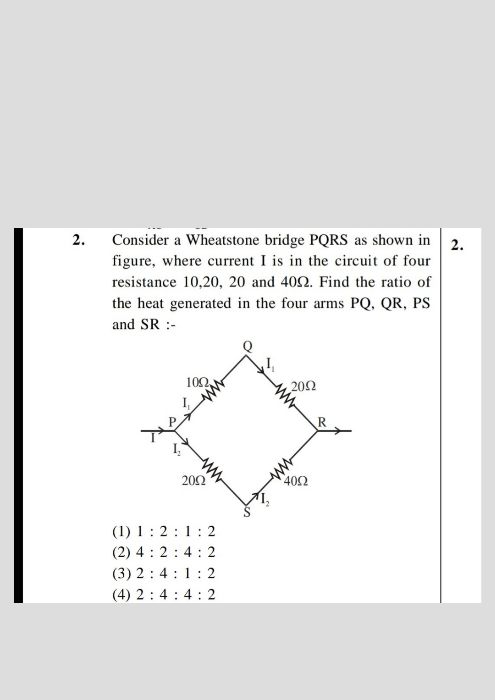
CBSE 12-science - Physics
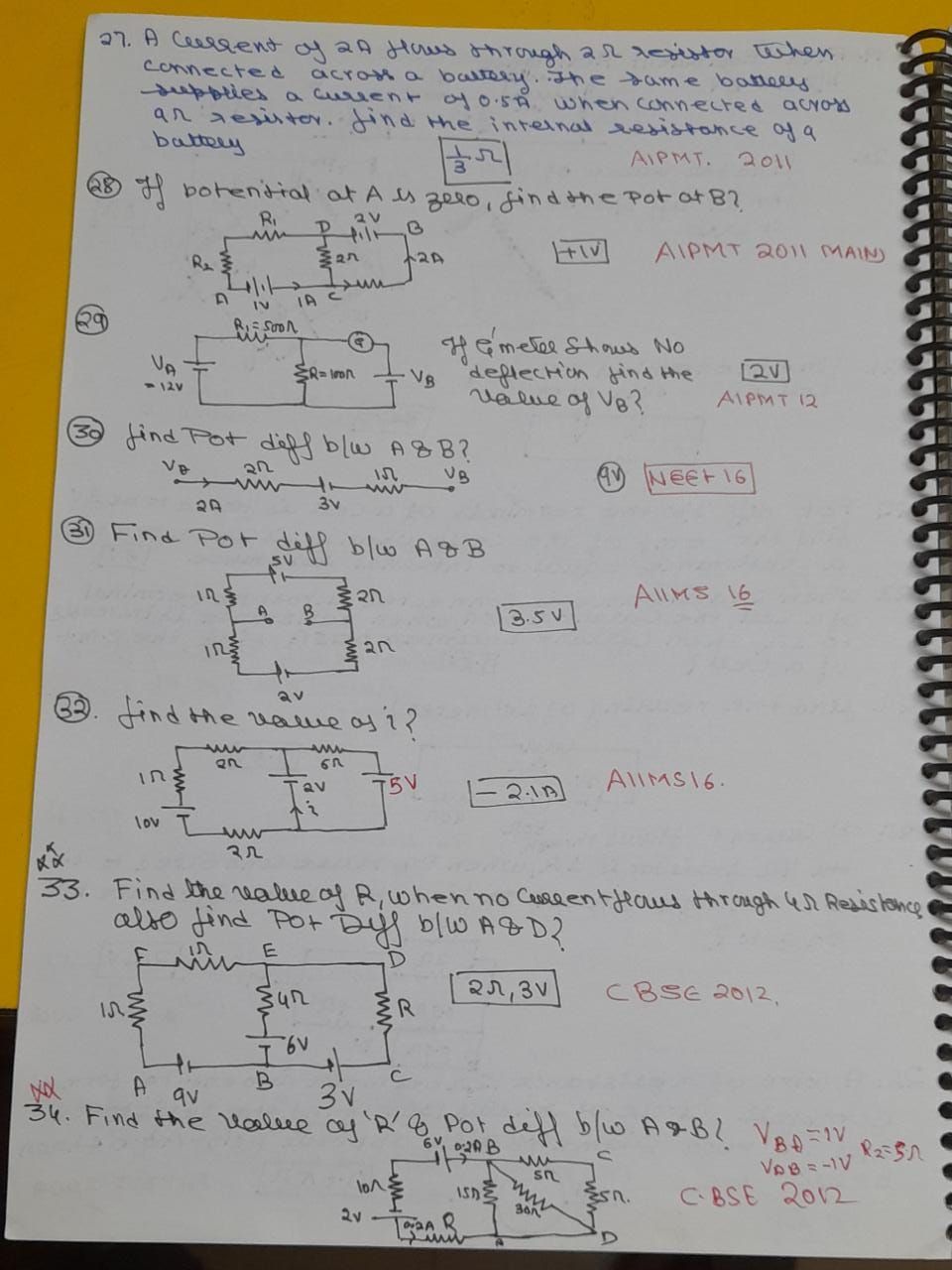
Asked by prakriti12oct | 28 Jul, 2019, 23:07: PM
CBSE 12-science - Physics
Asked by rejimoljohn123 | 01 Jan, 2019, 21:08: PM
CBSE 12-science - Physics
Asked by ayushmittal2424 | 09 Dec, 2018, 21:45: PM
CBSE 12-science - Physics
Asked by sd2021667 | 02 Dec, 2018, 21:09: PM
CBSE 12-science - Physics
Asked by rohitbansal39295 | 15 Nov, 2018, 21:00: PM
CBSE 12-science - Physics
Asked by my3shyll | 24 Sep, 2018, 19:33: PM