CBSE Class 11-science Answered

Asked by anusharma230703 | 11 Sep, 2020, 22:35: PM
In RUTHERFORD EXPT,
the assumptions were that the observed scattering of alpha particals came from single encounters with nuclei and assuming that the force was just the electrostatic rerpulsion.
Rutherford found scattering angle as a function of ingoing speed and impact parameteris given bu :
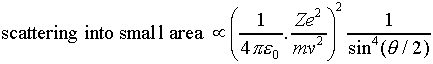 However when Aluminium foil was used ,although small scattering were following the rule while greater angle scattering didnt because in larger angle scattering which correspond to closer approach to the nuclei the partical was actually hitting the nucleus,that is size of the nucleus can be found bu finding the maximum angle for which inverse square of scattering formula worked.
However when Aluminium foil was used ,although small scattering were following the rule while greater angle scattering didnt because in larger angle scattering which correspond to closer approach to the nuclei the partical was actually hitting the nucleus,that is size of the nucleus can be found bu finding the maximum angle for which inverse square of scattering formula worked.
Answered by Ravi | 13 Sep, 2020, 10:34: AM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by dhondesainath | 28 Sep, 2021, 07:45: AM
CBSE 11-science - Chemistry
Asked by jaidawra48 | 13 Aug, 2021, 18:30: PM
CBSE 11-science - Chemistry
Asked by aayushiyadav408 | 12 Jul, 2021, 15:26: PM
CBSE 11-science - Chemistry
Asked by anandkumar10.12.96 | 28 Dec, 2020, 14:34: PM
CBSE 11-science - Chemistry
Asked by fizajain21 | 18 Dec, 2020, 12:46: PM
CBSE 11-science - Chemistry
Asked by darshanabhamare72 | 16 Oct, 2020, 18:31: PM
CBSE 11-science - Chemistry
Asked by brahampreetkaur818 | 15 Oct, 2020, 22:13: PM
CBSE 11-science - Chemistry
Asked by anish.4006 | 18 Sep, 2020, 20:00: PM





