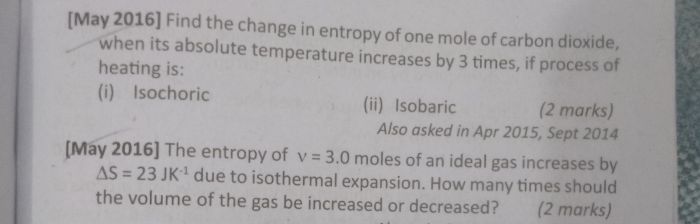JEE Class main Answered
if the ratio of the specific heats of steam is 1.33 and R=8312j/k mole k the molar heat capacities of steam at constant pressure and constant volume is
Asked by ghrushi3 | 02 Nov, 2023, 10:05: PM
Ratio of specific heats = 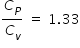
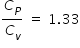
Where Cp is constant pressure specific heat , Cv is constant volume specific heat .
Hence we get 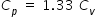 ...............................(1)
...............................(1)
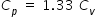 ...............................(1)
...............................(1)Cp , Cv and universal gas constant R are related as
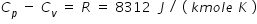 .................................(2)
.................................(2)From eqn. (1) and eqn.(2) , we get
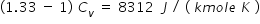
Hence , we get , Cv = 8312 / 0.33 J / ( kmole K ) = 25188 J / (kmole K )
From eqn.(1) , Cp = 33500 J/ (kmole K )
Answered by Thiyagarajan K | 02 Nov, 2023, 11:16: PM
JEE main - Physics
Asked by hridayjayaram085 | 12 Jan, 2024, 05:50: PM
JEE main - Physics
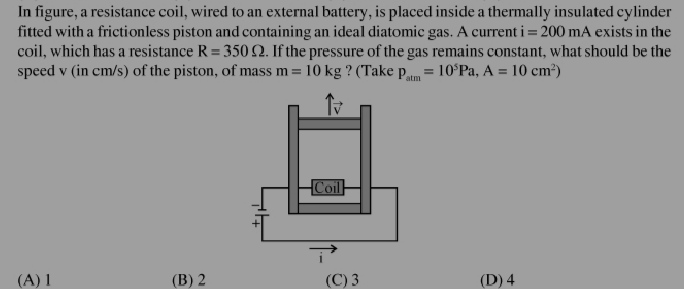
Asked by ashainy91829 | 06 Nov, 2023, 01:08: PM
JEE main - Physics
Asked by ghrushi3 | 02 Nov, 2023, 10:05: PM
JEE main - Physics
Asked by samarthghogare | 06 May, 2023, 11:17: AM
JEE main - Physics
Asked by aadityakumar0603 | 06 Mar, 2023, 10:03: PM
JEE main - Physics
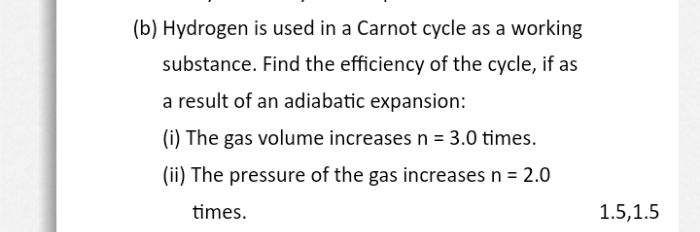
Asked by manvirsingh2242 | 21 Jun, 2022, 04:35: PM
JEE main - Physics
Asked by manvirsingh2242 | 20 Jun, 2022, 12:32: PM
JEE main - Physics
Asked by manvirsingh2242 | 18 Jun, 2022, 06:27: PM
JEE main - Physics
Asked by manvirsingh2242 | 11 Jun, 2022, 09:16: AM