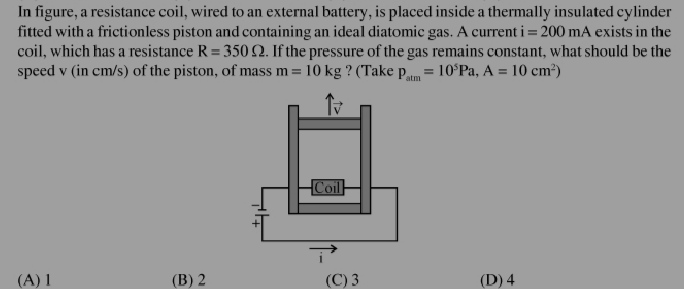
JEE Class main Answered
plz help me in solving my doubt
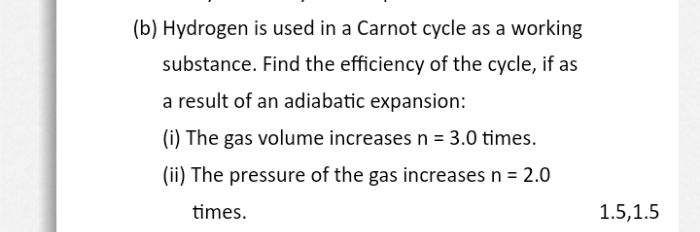
Asked by manvirsingh2242 | 11 Jun, 2022, 09:16: AM
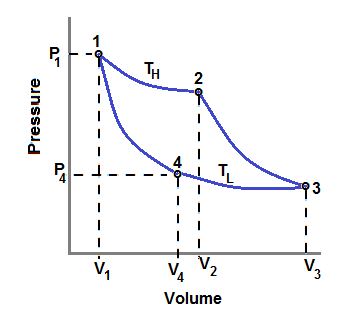
At state-1 , we have , P1 V1 = n R TH ............................. (1)
where P is pressure, V is volume, n is number of moles, R is universal gas constant and TH is source temperature
At state-4 , we have , P4 V4 = n R TL ...........................(2)
Where TL is sink temperature
By dividing eqn.(1) by eqn.(2), we get
( P1 / P4 ) ( V1 / V4 ) = TH / TL
We are given that ( P1 / P4 ) = 2 and ( V1 / V4 ) = 1/3
Hence , we get , TH / TL = 2/3
actually we should get , TH / TL > 1 because source temperature is greater than sink temperature.
Hence the given pressure ratio is wrong , it shoud be greater than 3
Efficiency η = 1 - ( TL / TH )
Answered by Thiyagarajan K | 11 Jun, 2022, 15:44: PM
JEE main - Physics
Asked by rambabunaidu4455 | 03 Oct, 2024, 16:03: PM
JEE main - Physics
Asked by yashu22022006 | 25 May, 2024, 09:13: AM
JEE main - Physics
Asked by pataiyalalit02 | 19 May, 2024, 16:53: PM
JEE main - Physics
Asked by hridayjayaram085 | 12 Jan, 2024, 17:50: PM
JEE main - Physics
Asked by ashainy91829 | 06 Nov, 2023, 13:08: PM
JEE main - Physics
Asked by ghrushi3 | 02 Nov, 2023, 22:05: PM
JEE main - Physics
Asked by samarthghogare | 06 May, 2023, 11:17: AM
JEE main - Physics
Asked by aadityakumar0603 | 06 Mar, 2023, 22:03: PM
JEE main - Physics
Asked by manvirsingh2242 | 21 Jun, 2022, 16:35: PM