JEE Class main Answered
Calculate the increase in entropy of 1 kg of ice when it is converted into steam. Given the specific heat of water is 1 kcal kg oC, latent heat of ice is 80 kcal/kg and the latent heat of steam is 540 k cal/kg.
Asked by ashainy91829 | 06 Nov, 2023, 01:08: PM
Latent heat of melting of ice , Lf = 80 kcal/kg = 80 × 4.184 kJ/ kg = 335 kJ/kg
Let m = 1 kg be the mass of ice
Change in entropy ΔS1 for melting of ice at temperature 273 K is
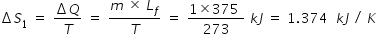
Specific heat of water, Cp = 1 kCal = 4.184 kJ/kg
Change in entropy ΔS2 for increasing the water temperature from 0o C ( 273 K ) to 100oC ( 373 K ) is
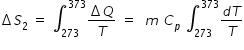
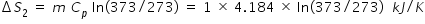
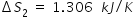
Latent heat of vapouraisation of water , Lv = 540 kCal / kg = 540 × 4.184 kJ / kg = 2259 kJ/kg
Change in entropy ΔS3 for converting water at boiling point to steam is
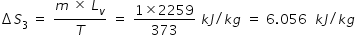
Total entropy ΔS is
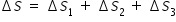
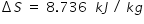
Answered by Thiyagarajan K | 06 Nov, 2023, 05:31: PM
JEE main - Physics
Asked by hridayjayaram085 | 12 Jan, 2024, 05:50: PM
JEE main - Physics
Asked by ashainy91829 | 06 Nov, 2023, 01:08: PM
JEE main - Physics
Asked by ghrushi3 | 02 Nov, 2023, 10:05: PM
JEE main - Physics
Asked by samarthghogare | 06 May, 2023, 11:17: AM
JEE main - Physics
Asked by aadityakumar0603 | 06 Mar, 2023, 10:03: PM
JEE main - Physics
Asked by manvirsingh2242 | 21 Jun, 2022, 04:35: PM
JEE main - Physics
Asked by manvirsingh2242 | 20 Jun, 2022, 12:32: PM
JEE main - Physics
Asked by manvirsingh2242 | 18 Jun, 2022, 06:27: PM
JEE main - Physics
Asked by manvirsingh2242 | 11 Jun, 2022, 09:16: AM