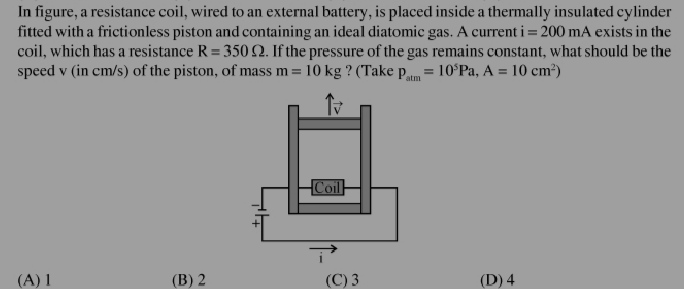
JEE Class main Answered
solve doubts
Asked by manvirsingh2242 | 18 Jun, 2022, 18:27: PM
Carbon-di-oxide is non-linear tri-atomic molecule that has 6 degrees of freedom
( 3 translation + 3 rotation )
Constant volume specific heat Cv of carbon-di-oxide gas is given as
Cv = ( 6/2) R = 3 R
where R is universal gas constant
Constant pressure specific heat Cp of carbon-di-oxide gas is given as
Cp = Cv + R = 4 R
Change in entropy ( ΔS )v for isochoric process ( constant volume ) is given as
( ΔS )v = n Cv ln( Tf / Ti )
where n is number of moles , Tf is final absolute temperature and Ti is absolute temperature
( ΔS )v = 3 R ln( 3 ) = 3 × 8.314 × ln(3) = 27. 4 J / K
Change in entropy ( ΔS )p for isobaric process ( constant pressure ) is given as
( ΔS )p = n Cp ln( Tf / Ti )
( ΔS )p = 4 R ln( 3 ) = 4 × 8.314 × ln(3) = 36.5 J / K
Answered by Thiyagarajan K | 18 Jun, 2022, 20:47: PM
JEE main - Physics
Asked by rambabunaidu4455 | 03 Oct, 2024, 16:03: PM
JEE main - Physics
Asked by yashu22022006 | 25 May, 2024, 09:13: AM
JEE main - Physics
Asked by pataiyalalit02 | 19 May, 2024, 16:53: PM
JEE main - Physics
Asked by hridayjayaram085 | 12 Jan, 2024, 17:50: PM
JEE main - Physics
Asked by ashainy91829 | 06 Nov, 2023, 13:08: PM
JEE main - Physics
Asked by ghrushi3 | 02 Nov, 2023, 22:05: PM
JEE main - Physics
Asked by samarthghogare | 06 May, 2023, 11:17: AM
JEE main - Physics
Asked by aadityakumar0603 | 06 Mar, 2023, 22:03: PM
JEE main - Physics
Asked by manvirsingh2242 | 21 Jun, 2022, 16:35: PM