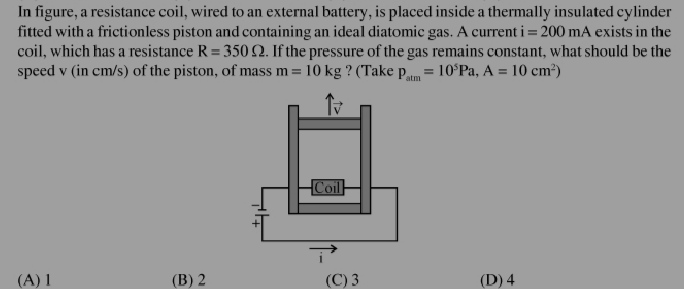
JEE Class main Answered
solve my doubt

Asked by manvirsingh2242 | 21 Jun, 2022, 16:35: PM
Let us consider that entropy S is a function of volume V and temperature T
Then we have ,
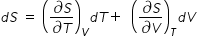 .......................(1)
.......................(1)During vapouraisation of water to steam, temperature is constant ,
i.e, dT=0 ......................(2)
By Maxwell's thermodynamics relation, we have
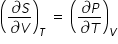 .......................(3)
.......................(3)Using eqn.(2) and eqn.(3) , we rewrite eqn.(1) as
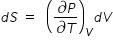
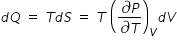 ......................(4)
......................(4)where dQ = m × L is heat absorbed during vapouraisation process, m is mass of water
and L is Latent heat of vapouraisation .
Hence eqn.(4) becomes ,
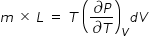
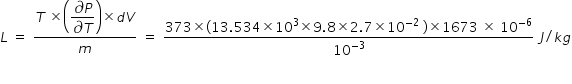
( bracketed term in numerator of above expression is ∂P = ρ g h , where ρ is density of mercury ,
g is acceleration due to gravity and h is height given for the measure of pressure )
From above expression, we get latent heat of vapouraistation of water L = 2.235 × 106 J/kg
Answered by Thiyagarajan K | 21 Jun, 2022, 20:09: PM
JEE main - Physics
Asked by rambabunaidu4455 | 03 Oct, 2024, 16:03: PM
JEE main - Physics
Asked by yashu22022006 | 25 May, 2024, 09:13: AM
JEE main - Physics
Asked by pataiyalalit02 | 19 May, 2024, 16:53: PM
JEE main - Physics
Asked by hridayjayaram085 | 12 Jan, 2024, 17:50: PM
JEE main - Physics
Asked by ashainy91829 | 06 Nov, 2023, 13:08: PM
JEE main - Physics
Asked by ghrushi3 | 02 Nov, 2023, 22:05: PM
JEE main - Physics
Asked by samarthghogare | 06 May, 2023, 11:17: AM
JEE main - Physics
Asked by aadityakumar0603 | 06 Mar, 2023, 22:03: PM
JEE main - Physics
Asked by manvirsingh2242 | 21 Jun, 2022, 16:35: PM