CBSE Class 12-science Answered
how many ml of 0.1M HCL are required to react completely with 1g mixture of and NaHCO3 containing equimolar amounts of both?
Asked by rakeshraghav33 | 18 Sep, 2018, 16:22: PM
Question:
How many ml of 0.1M HCL are required to react completely with a 1g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?
Solution:
Molarity of HCl = 0.1 M Na2CO3 and NaHCO3 = 1 g
The weight of mixture of
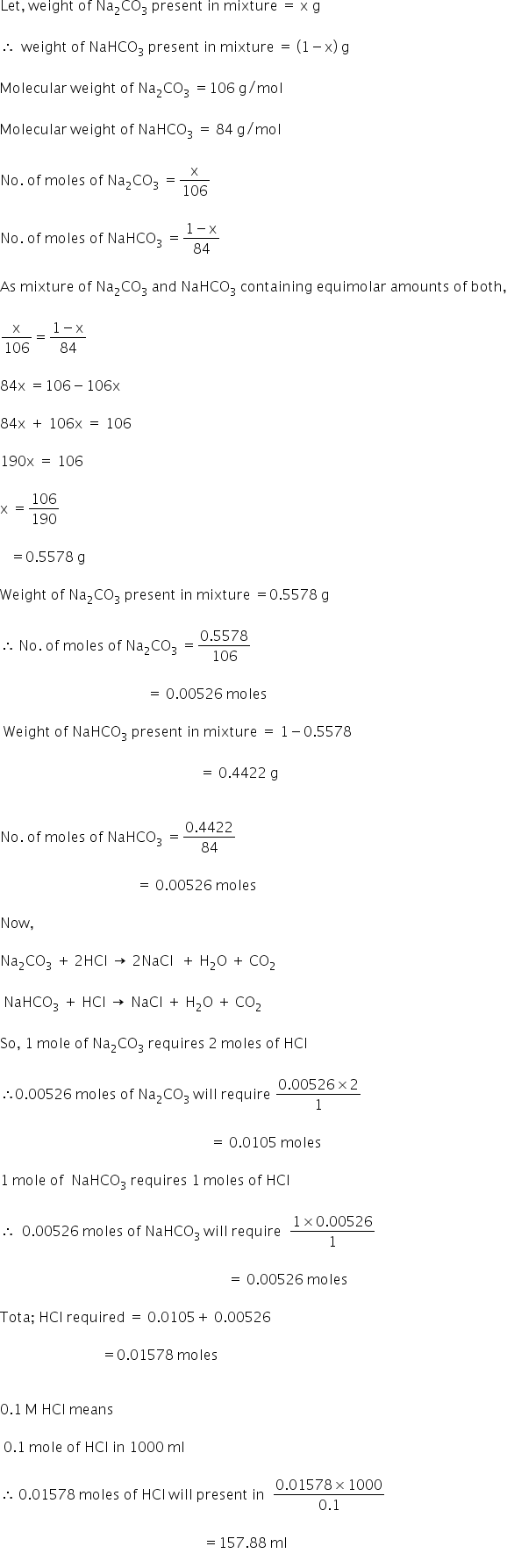
The volume HCl required is 157.88 ml.
Answered by Varsha | 19 Sep, 2018, 11:43: AM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 16:01: PM
CBSE 12-science - Chemistry
Asked by ayazanwarneet | 03 Jan, 2023, 22:58: PM
CBSE 12-science - Chemistry
Asked by gopirammeghwal78 | 05 Aug, 2021, 08:18: AM
CBSE 12-science - Chemistry
Asked by msambyal307 | 30 May, 2021, 14:03: PM
CBSE 12-science - Chemistry
Asked by nishchaymakhija115 | 11 Sep, 2019, 14:31: PM
CBSE 12-science - Chemistry
Asked by pandeyn1604 | 29 May, 2019, 09:16: AM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 18 Sep, 2018, 16:22: PM
CBSE 12-science - Chemistry
Asked by Avadhut Katkar | 14 Sep, 2018, 12:37: PM
CBSE 12-science - Chemistry
Asked by minipkda | 21 May, 2018, 22:19: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 16:00: PM

