CBSE Class 12-science Answered
At what partial
pressure oxygen will have a solubility of 0.06 gram per litre in water at 293 Kelvin Henry's law constant of oxygen in water at 303 kelvin is 46.82 k bar assume the density of the solution to the same as that of water
Asked by pandeyn1604 | 29 May, 2019, 09:16: AM
Given:
Solubility of O2 = 0.06 gram/litre
KH = 46.82 kbar
= 46.82 × 103 bar
Mass of 1 L of water = 1000 gm
Mass of solvent = 1000 - 0.06
≈ 1000 gm
No. of moles of water
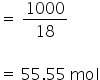
No. of moles of O2
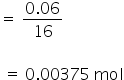
Mole fraction of O2
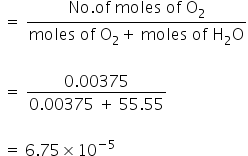
We have,
PO2 = KH × ΧO2
= 46.82 × 103 × 6.75 × 10−5
= 3.160 bar
The partial pressure of O2 is 3.16 bar.
Answered by Varsha | 29 May, 2019, 12:01: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 16:01: PM
CBSE 12-science - Chemistry
Asked by ayazanwarneet | 03 Jan, 2023, 22:58: PM
CBSE 12-science - Chemistry
Asked by gopirammeghwal78 | 05 Aug, 2021, 08:18: AM
CBSE 12-science - Chemistry
Asked by msambyal307 | 30 May, 2021, 14:03: PM
CBSE 12-science - Chemistry
Asked by nishchaymakhija115 | 11 Sep, 2019, 14:31: PM
CBSE 12-science - Chemistry
Asked by pandeyn1604 | 29 May, 2019, 09:16: AM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 18 Sep, 2018, 16:22: PM
CBSE 12-science - Chemistry
Asked by Avadhut Katkar | 14 Sep, 2018, 12:37: PM
CBSE 12-science - Chemistry
Asked by minipkda | 21 May, 2018, 22:19: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 16:00: PM

