CBSE Class 11-science Answered
how do we get to know if a molecule is polar or non polar in nature?what is an electron cloud?
Asked by nand DAVE | 29 Jul, 2014, 07:15: PM
Dear nand030599@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
however, answer to your first query is,
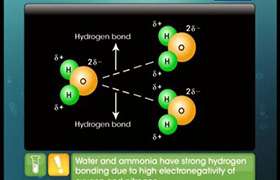
A polar molecule has a net dipole as a result of the opposing charges (i.e. having partial positive and partial negative charges) from polar bonds arranged asymmetrically.
Water (H2O) is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. The dipoles do not cancel out resulting in a net dipole. Due to the polar nature of the water molecule itself, polar molecules are generally able to dissolve in water.
A molecule may be non-polar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule.
Most non-polar molecules are water-insoluble (hydrophobic) at room temperature. However, many non-polar organic solvents, such as turpentine, are able to dissolve polar substances.Regards
Topperlearning Team.
Topperlearning Team.
Answered by Hanisha Vyas | 30 Jul, 2014, 11:56: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by neerudhawanbpsmv | 28 Jun, 2021, 09:22: AM
CBSE 11-science - Chemistry
Asked by swatipuspapatel | 10 Jun, 2020, 06:19: PM
CBSE 11-science - Chemistry
Asked by vanyashree106 | 13 Dec, 2019, 09:38: PM
CBSE 11-science - Chemistry
Asked by Asif.k1992 | 12 Sep, 2019, 02:41: PM
CBSE 11-science - Chemistry
Asked by ajitbhot73 | 25 Aug, 2019, 11:52: AM
CBSE 11-science - Chemistry
Asked by design1.bharatifire | 21 Jun, 2019, 06:35: PM
CBSE 11-science - Chemistry
Asked by anitabahuguna | 04 May, 2019, 09:57: PM
CBSE 11-science - Chemistry
Asked by Jatinaa786 | 19 Sep, 2018, 02:53: AM
CBSE 11-science - Chemistry
Asked by arjunshrivastav668 | 17 Mar, 2018, 10:21: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM