CBSE Class 11-science Answered
How do the alkali and alkaline earth metals react with oxygen?
Asked by Topperlearning User | 28 Jun, 2016, 13:35: PM
The alkali metals on exposure to air or oxygen burn vigorously, forming oxides on the surface of the metals. Lithium forms monoxide (Li2O), sodium forms the peroxide (Na2O2) and the other elements form super-oxides.
(MO2: M = K, Rb, Cs).
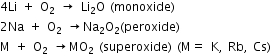
Alkaline earth metals react with air or oxygen slowly upon heating (being less electropositive than alkali metals) to form oxides (MO), except Ba and Ra, which form peroxides.
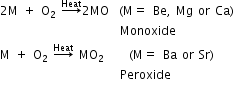
Answered by | 28 Jun, 2016, 15:35: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreyasharma94.11dga | 23 Mar, 2022, 23:42: PM
CBSE 11-science - Chemistry
Asked by mrassam2711 | 15 Jan, 2021, 23:54: PM
CBSE 11-science - Chemistry
Asked by sg908580 | 21 Jan, 2020, 21:16: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 13:37: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 13:37: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 28 Jun, 2016, 13:35: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 12 Aug, 2014, 13:43: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM




