CBSE Class 10 Answered
Explain the formation of CaF2.
Asked by Topperlearning User | 24 Jul, 2017, 14:20: PM
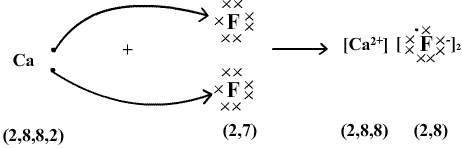
Atomic Number of Ca is 20.
Electronic Configuration of Ca : 2, 8, 8, 2
When Calcium loses two electrons, it becomes Ca2+ and attains stable electronic arrangement of argon.
Now,
Ca → Ca2+ + 2e-
Atomic Number of F = 9
Electronic Configuration of F : 2, 7
Fluorine on gaining one electron forms an anion F- and attains stable electronic arrangement of neon.
F + e- → F-
The oppositely charged ions are held together by strong electrostatic forces of attraction as shown :-
Answered by | 24 Jul, 2017, 16:20: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by iashish0605 | 16 Oct, 2020, 13:53: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 21 Jun, 2019, 15:07: PM
CBSE 10 - Chemistry
Asked by Topperlearning User | 12 Sep, 2014, 17:40: PM
CBSE 10 - Chemistry
Asked by Topperlearning User | 29 Jan, 2015, 10:20: AM
CBSE 10 - Chemistry
Asked by Topperlearning User | 12 Sep, 2014, 17:51: PM
CBSE 10 - Chemistry
Asked by Topperlearning User | 21 May, 2014, 12:26: PM
CBSE 10 - Chemistry
Asked by Topperlearning User | 24 Jul, 2017, 14:20: PM
CBSE 10 - Chemistry
Asked by Topperlearning User | 24 Jul, 2017, 14:20: PM
CBSE 10 - Chemistry
Asked by Topperlearning User | 26 Jun, 2014, 13:34: PM
CBSE 10 - Chemistry
Asked by Topperlearning User | 29 Jan, 2015, 10:18: AM