CBSE Class 12-science Answered
Explain B.pt.elevation and F.pt.depression?
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 17:52: PM
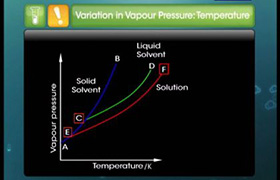
Boiling Point Elevation- Elevation in boiling point refers to increase in boiling boint of solvent upon the addition of solute. When a non-volatile solute is added then resulting solution has a higher boiling point then pure solvent. For example- Boiling point of Salt+water solution is more than pure water.
Freezing Point Depression-Freezing Point Depression refers to lowering of the freezing point of solvents upon the adition of solute. When a non-volatile solute is added then resulting solution has a lower freezing point then pure solvent.
To know more about boiling point elevation and freezing point depression, Follow the given link-
Answered by Ravi | 15 Jul, 2020, 18:41: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by chetanrakshit06 | 05 May, 2024, 14:51: PM
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 20:21: PM
CBSE 12-science - Chemistry
Asked by vekariyaparth61 | 16 May, 2022, 16:33: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 13:27: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 13:25: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 13:20: PM
CBSE 12-science - Chemistry
Asked by sshashu993 | 25 Jul, 2020, 08:02: AM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 17:52: PM
CBSE 12-science - Chemistry
Asked by sharmasherryal | 25 May, 2020, 09:54: AM