CBSE Class 11-science Answered
Calculate the number of Cl- ion in 100 ml of the solution which is formed by mixing of 200 ml of 2M NaCl(aq) , 800 ml 1M MgCl2 (aq) solution and 1 litre 5.3× 10^ -4 M CCl4 (aq) solution ?
Asked by arunavamitra50 | 29 May, 2019, 12:42: PM
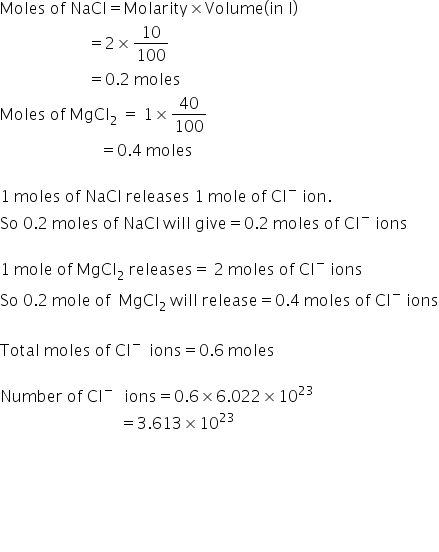
CCl4 is a nonpolar compound which does not release Cl ion in solution.
Only NaCl and MgCl2 will release Cl ion.
NaCl, MgCl2 and CCl4 will have ratio of
200: 800 : 1000
1:4:5
When we are taking 100ml sample,
ratio will be same because mixture is uniformly distributed
So i 100ml, NaCl will have=10 ml
MgCl2 will have=40 ml
CCl4 will have=50ml
Answered by Ravi | 30 May, 2019, 05:50: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM







