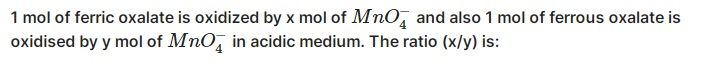CBSE Class 11-science Answered
50.0kg of N2[g] and 10.0 kg of H2 [g] are mixed to produce NH3[g] formed.Identify the limiting reagent in production of NH3 in this situaton
Asked by virubloda6 | 21 May, 2019, 08:39: AM
Given:
Mass of N2 = 50 kg
= 50 × 103 gm
No. of moles of N2
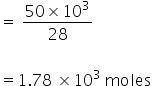
Mass of H2 = 10 kg
= 10 × 103 gm
No. of moles of H2
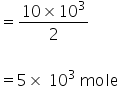
Formation of ammonia;
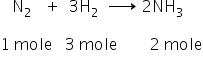
From reaction, 1 mole of N2 requires 3 moles H2.
So 1.78× 103 mole of N2 will require (1.78×103 ×3) = 5.34 ×103 moles of H2.
Here 5×103 moles of H2 are given, therefore H2 is the limiting reagent.
Now 5×103 moles of H2 will give,
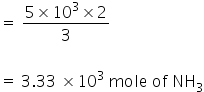
Therfore the amount of NH3 produced is 3.33×103 mole.
Answered by Varsha | 21 May, 2019, 11:52: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreekrishnampatil | 27 Apr, 2024, 10:31: PM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM