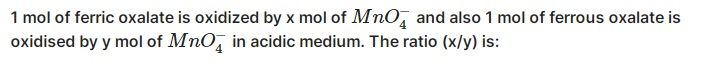CBSE Class 11-science Answered
26.8 g Na2SO4.nH2O has 12.6 g water. Find the value of n.
Asked by Anil | 15 May, 2017, 12:33: PM
Na2SO4 x n H2O
Molar mass of salt = (142 + 18n)
Mass of water = 12.6/26.8 x (142 + 18n)
18 n = 12.6/26.8 (142 + 18n)
n = 7
Answered by Vaibhav Chavan | 15 May, 2017, 12:50: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreekrishnampatil | 27 Apr, 2024, 10:31: PM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM