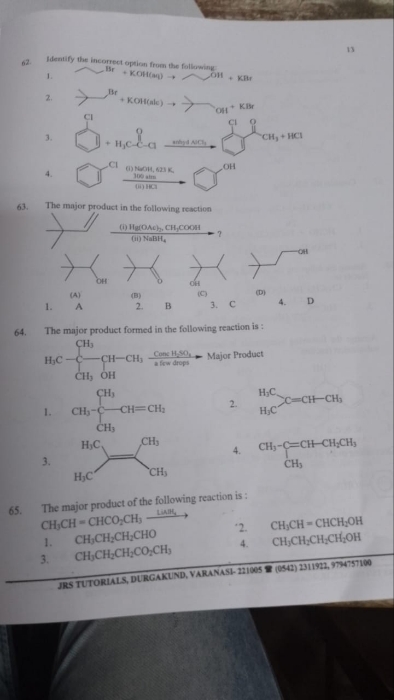
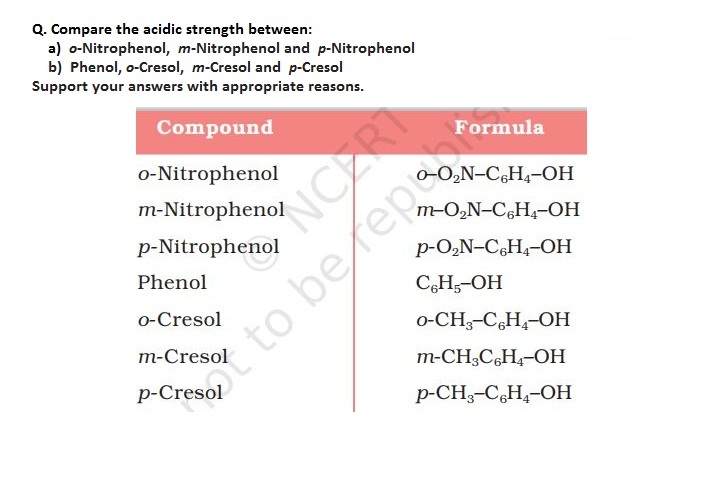
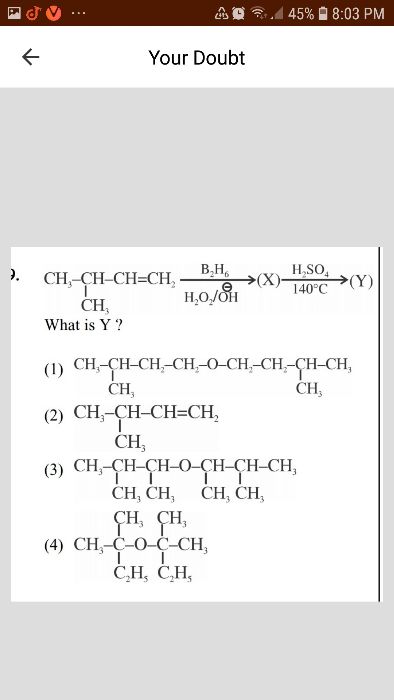
CBSE Class 12-science Answered
Phenols may be considered to be a resonance hybrid of the five contributing structures. as a result of resonance, the oxygen atom acquires a partial positive charge. this weakens the O-H bond and thus facilitates the release of a proton.
The phenoxide ion formed after removal of hydrogen ion also exist as a resonance hybrid of five contributing structures. But the phenoxide ion is better stabilised than phenol .However, in case of alcohol, both alcohol molecule and alkoxide ion are represented by one structure each and there is no resonance. The alkoxide ion due to presence of a formal negative charge on it has greater energy and is less stable than alcohol. Thus phenol is more acidic than alcohol.