CBSE Class 11-science Answered
URGENT!
Dear expert,
Pls help me out with this question with a proper solution:
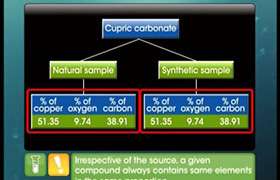
If the atomic mass unit 'u' were defined to be 1/5th of the mass of Carbon -12 isotopes what will be the atomic mass of nitrogen on carbon scale?(atomic weight of N on conventional scale= 14)
Asked by pratikshyadashrkl | 13 May, 2020, 08:29: PM
First let's see conventional scale-
Atomic mass of C-12 is 12 u
Atomic mass of N is 14 u
In new scale-
atomic mass unit is defined as 1/5 mass of carbon. (suppose its symbol is u' )
new atomic mass unit becomes 1/5 of conventional mass unit.
So,
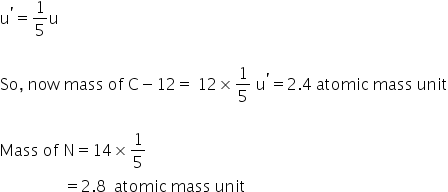
Answered by Ravi | 13 May, 2020, 09:18: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by dhrubapratimc | 13 Sep, 2023, 09:34: PM
CBSE 11-science - Chemistry
Asked by abhiabhishek842006 | 09 Jan, 2023, 08:34: PM
CBSE 11-science - Chemistry
Asked by Shashisinghkusum | 13 Jul, 2022, 02:44: PM
CBSE 11-science - Chemistry
Asked by shivanshiarora3457 | 09 Jun, 2022, 03:28: PM
CBSE 11-science - Chemistry
Asked by rishamariyam222 | 28 Sep, 2021, 09:18: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 08 Feb, 2021, 10:52: AM
CBSE 11-science - Chemistry
Asked by manteaditya8 | 08 Jan, 2021, 01:45: PM
CBSE 11-science - Chemistry
Asked by muanputhomte4 | 11 Nov, 2020, 09:01: PM
CBSE 11-science - Chemistry
Asked by pradeepprince858 | 07 Aug, 2020, 12:54: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 28 Jul, 2020, 11:03: AM