CBSE Class 11-science Answered
pls explain

Asked by shivanshiarora3457 | 09 Jun, 2022, 15:28: PM
Number of oxygen atoms in 22 g of CO2 .
Molar mass of CO2 =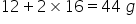
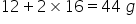
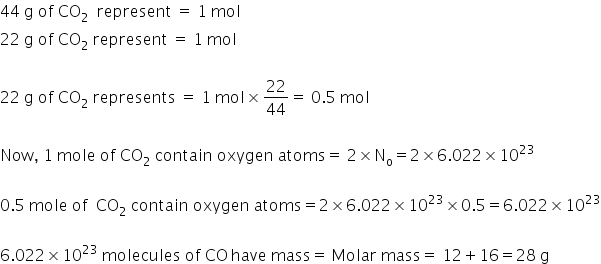
Answered by Ravi | 10 Jun, 2022, 12:33: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by dhrubapratimc | 13 Sep, 2023, 21:34: PM
CBSE 11-science - Chemistry
Asked by abhiabhishek842006 | 09 Jan, 2023, 20:34: PM
CBSE 11-science - Chemistry
Asked by shivanshiarora3457 | 09 Jun, 2022, 15:28: PM
CBSE 11-science - Chemistry
Asked by rishamariyam222 | 28 Sep, 2021, 21:18: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 08 Feb, 2021, 10:52: AM
CBSE 11-science - Chemistry
Asked by manteaditya8 | 08 Jan, 2021, 13:45: PM
CBSE 11-science - Chemistry
Asked by muanputhomte4 | 11 Nov, 2020, 21:01: PM
CBSE 11-science - Chemistry
Asked by pradeepprince858 | 07 Aug, 2020, 12:54: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 28 Jul, 2020, 11:03: AM