CBSE Class 11-science Answered
Hello sir,
please clarify my doubt.
can you explain me in detail about stoichiometry and limiting reagents with examples for each.
Thank you.
Asked by seeni2005 | 28 Jul, 2020, 11:03: AM
Dear Student,
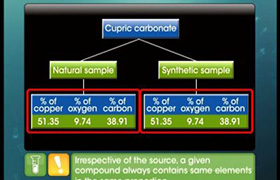
Please follow the given link to understand the concept and stoichiometry-
Now, Let's have a look on examples to solve the problems-
Question-1:
What is the amount of magnesium required to form 160 gram of magnesium oxide by burning magnesium in air?
Solution:
Mass of magnesium oxide = 160 gm
Reaction equation is;
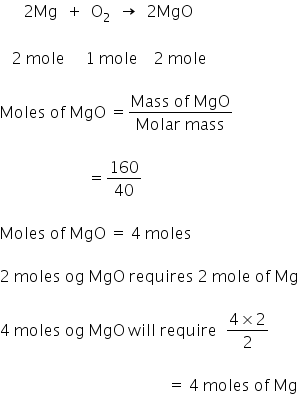 4 moles of magnesium is required to form 160 gm of magnesium oxide.
4 moles of magnesium is required to form 160 gm of magnesium oxide.
Question -2:
How many moles of Al2O3 will be formed when a mixture of 5.4g Al and 3.2g O2 is heated?
Kindly provide the answer.
Solution-

Answered by Ravi | 28 Jul, 2020, 18:14: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by dhrubapratimc | 13 Sep, 2023, 21:34: PM
CBSE 11-science - Chemistry
Asked by abhiabhishek842006 | 09 Jan, 2023, 20:34: PM
CBSE 11-science - Chemistry
Asked by shivanshiarora3457 | 09 Jun, 2022, 15:28: PM
CBSE 11-science - Chemistry
Asked by rishamariyam222 | 28 Sep, 2021, 21:18: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 08 Feb, 2021, 10:52: AM
CBSE 11-science - Chemistry
Asked by manteaditya8 | 08 Jan, 2021, 13:45: PM
CBSE 11-science - Chemistry
Asked by muanputhomte4 | 11 Nov, 2020, 21:01: PM
CBSE 11-science - Chemistry
Asked by pradeepprince858 | 07 Aug, 2020, 12:54: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 28 Jul, 2020, 11:03: AM