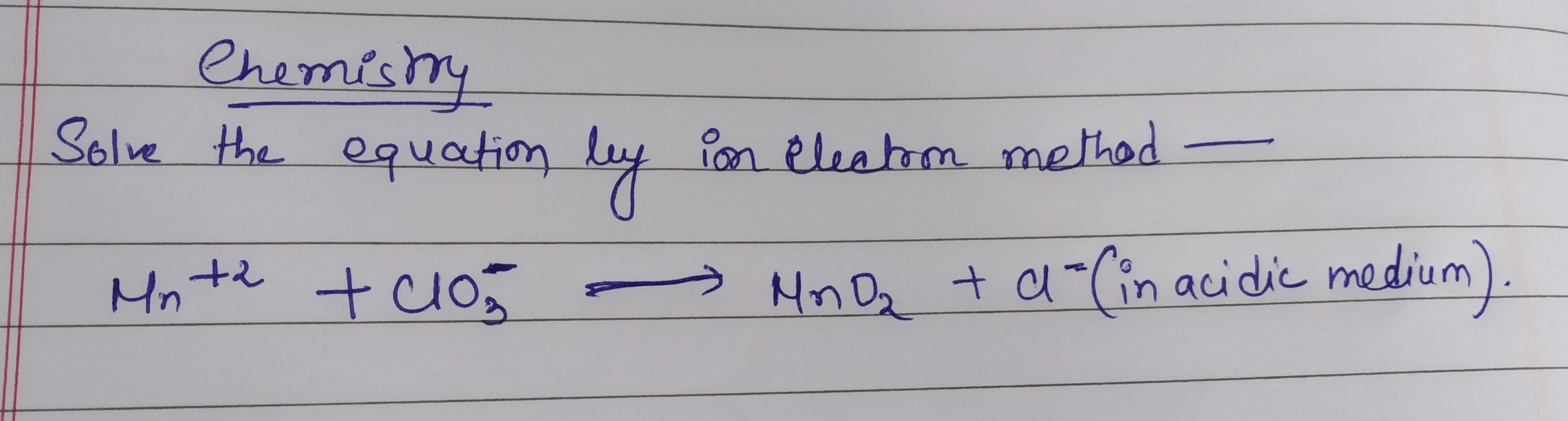
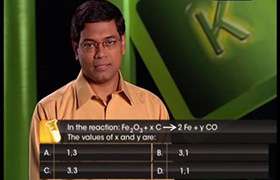CBSE Class 11-science Answered
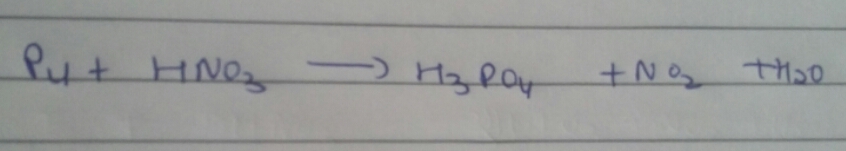
In most situations of balancing an equation, you are not told whether the reaction is redox or not.
In these circumstances, you can use a procedure called the oxidation number method.
The skeleton equation is:
P4 + HNO3 → H3PO4 + H2O + NO2
Step 2
The oxidation number of various atoms involved in the reaction.
0 +1 +5 -2 +1 +5 -2 +1 -2 +4 -2
P4 + HNO3 → H3PO4 + H2O + NO2
Step 3
For N oxidation number changes from +5 to +4 so it is reduced. For P oxidation number changes from 0 to +5 so it is oxidized. No change in oxidation number of O.
Step 4
Determine the net increase in oxidation number for the element that is oxidized and the net decrease in oxidation number for the element that is reduced.
For P 0 to +5 Net change = +5 ......... Oxidation
For N +5 to +4 Net change = -1 ..........Reduction
Step 5
Determine a ratio of oxidized to reduced atoms that would yield a net increase in oxidation number equal to the net decrease in oxidation number.
P atoms would yield a net increase in oxidation number of +5. (five electrons would be lost by four P atoms.). 1 N atom would yield a net decrease of -1. (One N atom would gain one electron.)
Thus the ratio of N atoms to C atoms is 5:1. But as we have P4 as a reactant hence the ratio will be changed (5 ×4) to 20:1
Step 6
To get the ratio identified in Step 5, add coefficients to the formulas which contain the elements whose oxidation number is changing.
and we will get the balanced equation,
P4 +20 HNO3 → 4H3PO4 + 20H2O + 4NO2