CBSE Class 11-science Answered
H2S + KMnO4 + H2SO4 → S+ MnSO4 + KHSO4 + H2O
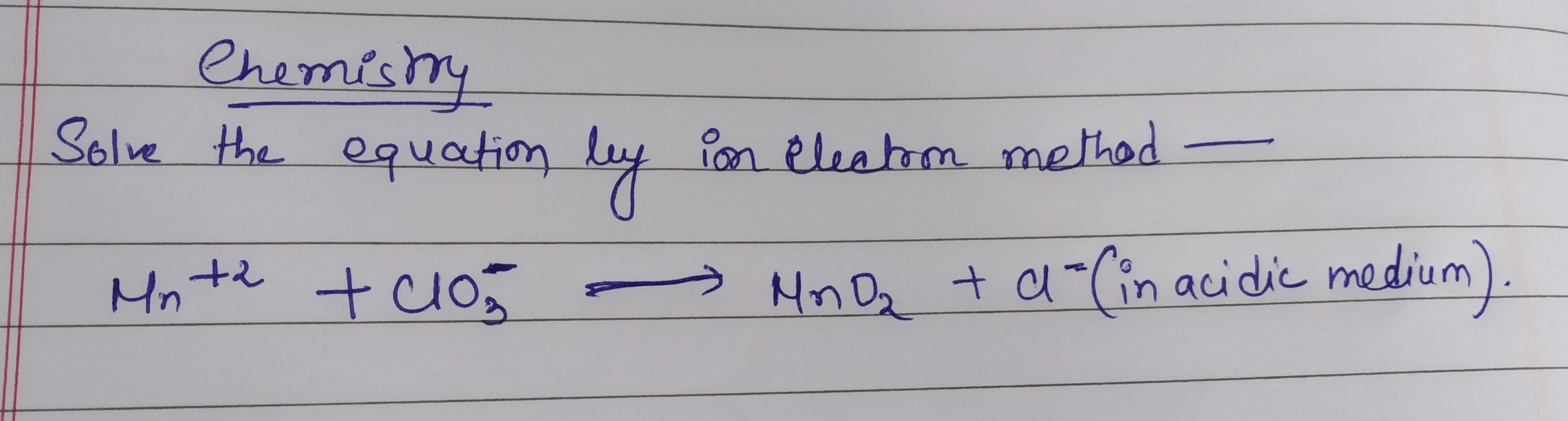
Balance by oxidation number method step by step explain please
Asked by govtsecschoolnayaganv051 | 18 Aug, 2018, 05:08: PM
Step 1
The skeleton equation is:
H2S + KMnO4 + H2SO4 → S+ MnSO4 + KHSO4 + H2O
Step 2
(a) The oxidation number of various atoms involved in the reaction.

(b) Identify and write out all the redox couple in reaction.

Step 3
Balance the atoms in each half reaction
a) Balance all other atoms except hydrogen an oxygen

b) Balance the charge:

c) Balance the oxygen atoms

Step 4: Make electron gain equivalent to electron lost.

Step 5: Add the half-reactions together.

Step 6 : Simplify the equation:

Answered by Ramandeep | 23 Aug, 2018, 10:19: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by indranibaudya | 03 Oct, 2020, 08:12: PM
CBSE 11-science - Chemistry
Asked by Punshibakhuraijam2015 | 22 Sep, 2019, 08:40: PM
CBSE 11-science - Chemistry
Asked by kkdmmdsd | 09 Jun, 2019, 04:27: PM
CBSE 11-science - Chemistry
Asked by dheerajmathpal374 | 15 Sep, 2018, 10:43: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Aug, 2018, 06:29: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 18 Aug, 2018, 05:08: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 14 Aug, 2018, 06:06: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 10:29: PM
CBSE 11-science - Chemistry
Asked by g_archanasharma | 22 Mar, 2018, 10:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM