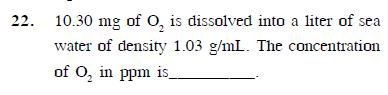Some Basic Concepts In Chemistry Free Doubts and Solutions
NEET - NEET - Chemistry - Some Basic Concepts in Chemistry
please answer this

NEET - NEET - Chemistry - Some Basic Concepts in Chemistry
please answer this
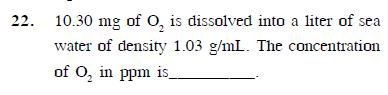
For Franchisee Enquiry
or
You are very important to us
For any content/service related issues please contact on this number
93219 24448 / 99871 78554
Mon to Sat - 10 AM to 7 PM