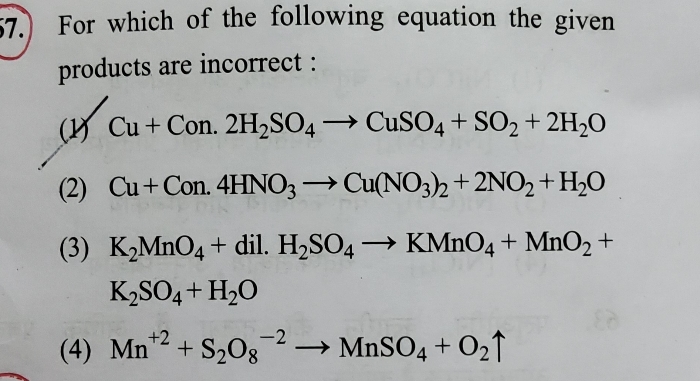
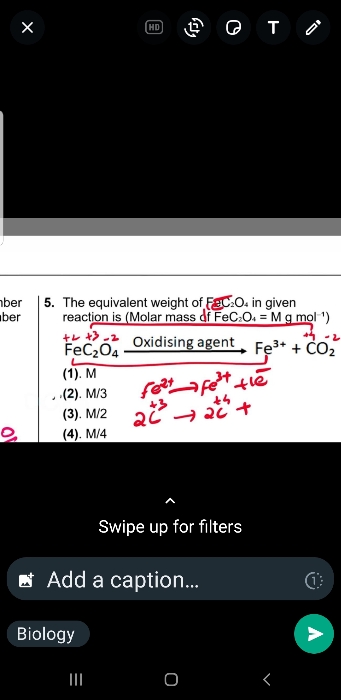
NEET Class neet Answered
please answer this

Asked by Prashant DIGHE | 29 Feb, 2020, 09:43: PM
Given:
Vapour density of metal chloride = 77
Molecular weight = 2 × 77 = 154 ..... (1)
Let the valency of element is x.
Atomic weight = Equi. wt × Valency
= 3× (x)
The formula of chloride is MClx
Mol. wt of chloride = 3x + 35.5x = 38.5x .... (2)
From eq(1) and (2)
38.5x = 154
x = 4
Atomic weight = 4× 3
= 12
Answered by Varsha | 01 Mar, 2020, 07:42: PM
Concept Videos
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by jetabanborthakur123 | 29 Mar, 2024, 07:05: PM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM