NEET Class neet Answered
Q128
Ans1

Asked by manasvijha | 24 Apr, 2019, 09:23: AM
Option (1) is correct.
Given:
Volume of NaOH = 20 ml
Molaity of NaOH = 0.1 M
Volume of CH3COOH = 30 ml
Molarity of CH3COOH = 0.2 M
CH3COOH + NaOH → CH3COONa + H2O
20 ml of 0.1 M NaOH contains 2 milli moles of NaOH
30 ml of 0.2 M CH3COOH contains 6 milli mole of CH3COOH
CH3COOH left unreacted = 6-2 = 4 millimole
CH3COONa formed = 2 milli mole
Volume of solution = 50 ml
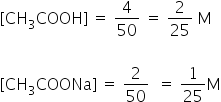
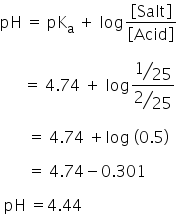
The pH of the resulting solution will be 4.44
Answered by Varsha | 24 Apr, 2019, 02:36: PM
Concept Videos
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by jetabanborthakur123 | 29 Mar, 2024, 07:05: PM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM