CBSE Class 12-science Answered
methods of preparations of aldehydes n ketones???
Asked by seema behera | 26 Jul, 2012, 04:11: PM
Oxidising alcohols to make aldehydes and ketones
The oxidising agent used in these reactions is normally a solution of sodium or potassium dichromate(VI) acidified with dilute sulphuric acid. If oxidation occurs, the orange solution containing the dichromate(VI) ions is reduced to a green solution containing chromium(III) ions.
The net effect is that an oxygen atom from the oxidising agent removes a hydrogen from the -OH group of the alcohol and one from the carbon to which it is attached.
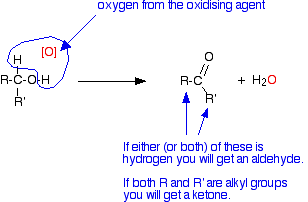
R and R' are alkyl groups or hydrogen. If at least one of these groups is a hydrogen atom, then you will get an aldehyde. If they are both alkyl groups then you get a ketone.
Answered by | 26 Jul, 2012, 07:22: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ukg8612 | 15 Apr, 2024, 07:36: PM
CBSE 12-science - Chemistry
Asked by ajayarchi | 08 Feb, 2024, 03:43: AM
CBSE 12-science - Chemistry
Asked by pallasriramulu9 | 24 Dec, 2023, 06:05: AM
CBSE 12-science - Chemistry
Asked by bsaheliya | 22 Dec, 2023, 09:53: PM
CBSE 12-science - Chemistry
Asked by ygarg8323 | 18 Apr, 2022, 12:47: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 30 Jun, 2021, 04:52: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 29 Jun, 2021, 08:36: AM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 28 Jun, 2021, 02:34: PM
CBSE 12-science - Chemistry
Asked by saimerala007 | 22 May, 2021, 02:08: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 31 Dec, 2020, 10:45: AM








