CBSE Class 11-science Answered
In the van der Waals equation, what do the constants 'a' and 'b' represent?
Asked by Topperlearning User | 21 Apr, 2015, 01:10: PM
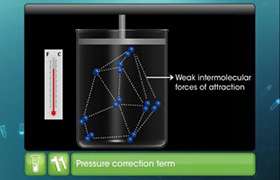
The constant 'a' gives the idea of the magnitude of attractive forces between the molecules of the gas and 'b' is the measure of effective volume occupied by the gas molecules in the van der Waals equation. It is also called co-volume or excluded volume.
Answered by | 21 Apr, 2015, 03:10: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 26 Jan, 2019, 12:57: PM
CBSE 11-science - Chemistry
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:19: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM