CBSE Class 11-science - Kinetic Theory Videos
States of Matter
This video explains the liquefaction of gases and critical behaviour of gases.
- if rms velocity nitrogen molecule is 5.15ms at 298K then a velocity of 10.30 ms will be prossesed at a temperature
- Give two evidences with explanation of molecular motion of gases.
-
Solve 5.24 sum plz
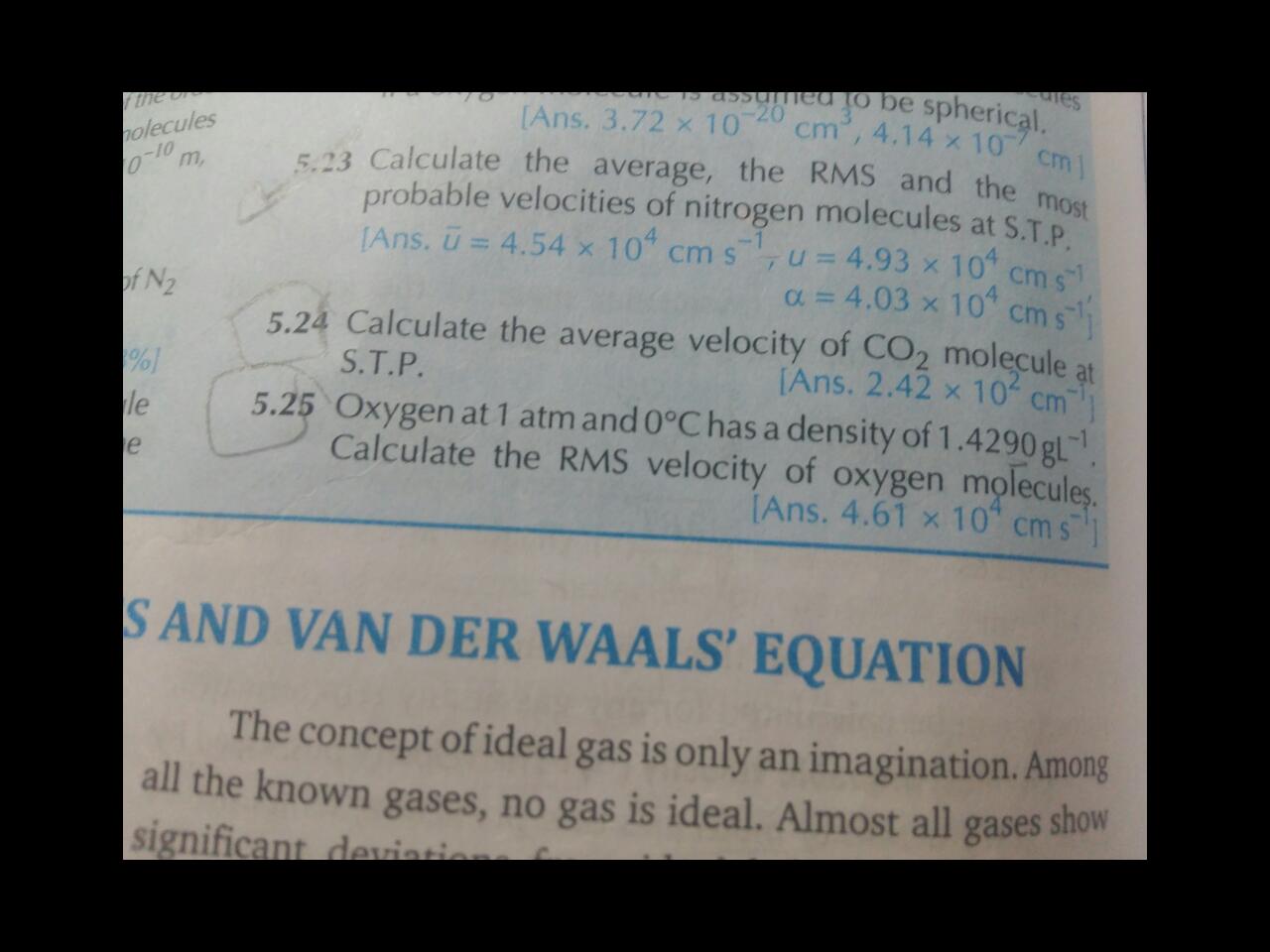
- State postulate of kinetic molecular
- 10th?
- How can we liquefy a gas, for which the value of α is 0?
- What is the relationship between three types of molecular speeds at a given temperature?
- What would happen if the molecular collisions in the kinetic theory are inelastic?
- Give reason for the high compressibility of gases on the basis of kinetic molecular theory?
- The average velocity of carbon dioxide gas at T1K and its most probable velocity at T2K is 9.0 x 104 cm sec-1. Calculate the value of T1 and T2?

