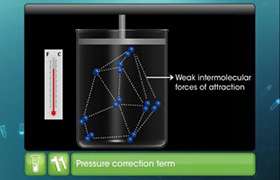
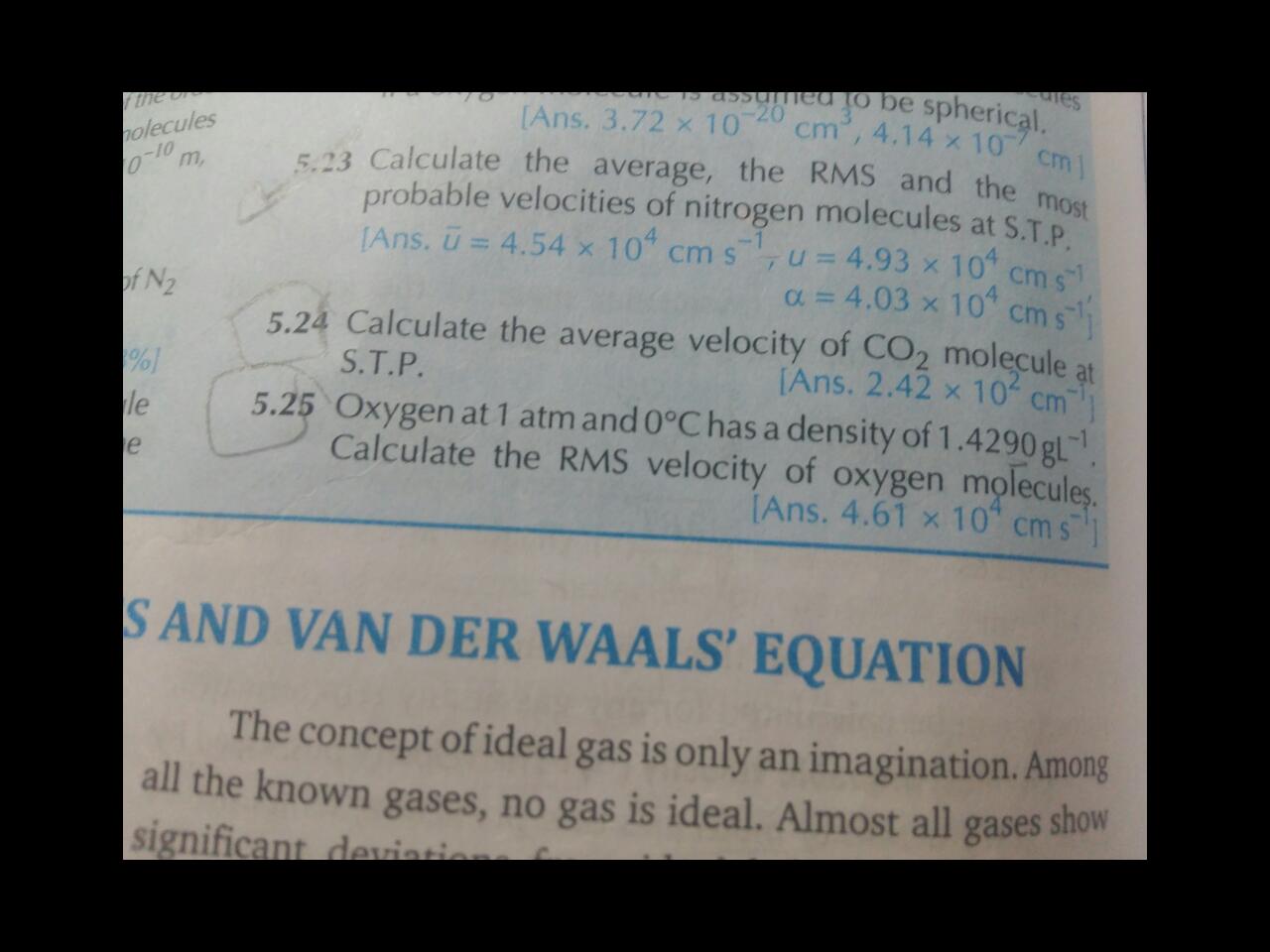CBSE Class 11-science Answered
10th?
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
Q.
The kinetic molecular theory attributes an average translational kinetic energy of 32RTN to each particle. What rms speed would a mist particle of mass 10−12 g have a root temperature (27∘C) according to kinetic theory of gases.
Solution:
Let the kinetic energy of particle be,

The rms velocity is 0.35 am/sec
Answered by Varsha | 05 Aug, 2018, 04:26: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 26 Jan, 2019, 12:57: PM
CBSE 11-science - Chemistry
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:19: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM