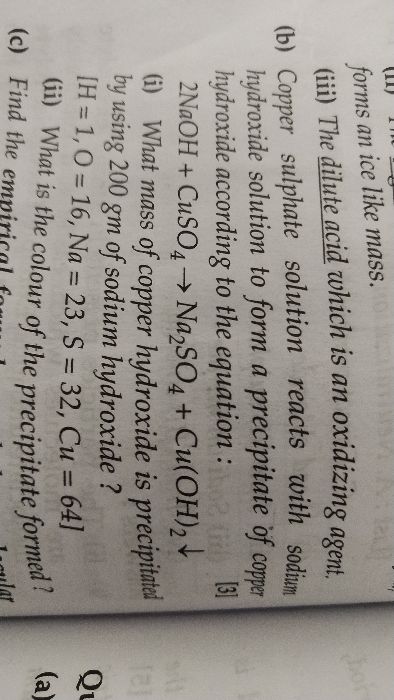ICSE Class 10 Answered
how to solve numericals on empirical formula and avogadro's law
Asked by aa26 | 27 Jan, 2019, 12:51: PM
- One mole is the amount of a substance that contains as many particles or entities as there are atoms in exactly 12 g (or 0.012 kg) of 12C.
- Following relations have given below can be summarized as
- One mole of atoms =
atoms = Gram atomic mass of an element.
- One mole of molecules =
molecules = Gram molecular mass of a substance.
- One mole of atoms =
- The concentration of a solution can be expressed in any of the following ways: Mass percent, mole fraction, molarity and molality.
- Molarity is the number of moles of solute in per liter of solution. The unit is moles per liter.
- Molality is the number of moles of solute present in 1 kg of solvent.
- A molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
- If the mass percent of various elements present in a compound is known, then its empirical formula can be determined.
- Where, n is a simple number and may have values 1, 2, 3….
- Following steps should be followed to determine the empirical formula of the compound:
- Step 1: Conversion of mass percent of various elements into grams.
- Step 2: Convert mass obtained in step 1 into the number of moles.
- Step 3: Divide the mole value obtained in step 2 by the smallest mole value (out of the mole value of various elements calculated).
- Step 4: In case the ratios are not whole numbers, they may be converted into whole numbers by multiplying by a suitable coefficient.
- Step 5: Write the empirical formula by mentioning the numbers after writing the symbols of respective elements.'
Answered by Ramandeep | 28 Jan, 2019, 12:22: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM