CBSE Class 12-science Answered
How do we find vant Hoff factor i
Asked by arnavvidudala20050 | 12 May, 2020, 10:41: AM
The relationship between the actual number of moles of solute added to form a solution and the apparent number as determined by colligative properties is called the van't Hoff factor (i).
It is used to express the extent of association or dissociation of solute. If association or dissociation of solute particles takes place in the solution, then
Let's understand it by few examples-
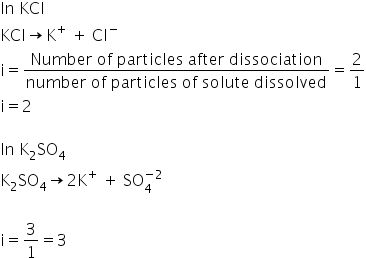
Answered by Ravi | 12 May, 2020, 05:47: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 04:01: PM
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by premkhare2006 | 24 Jan, 2024, 09:50: AM
CBSE 12-science - Chemistry
Asked by saritanohar22 | 13 Jan, 2024, 01:25: PM
CBSE 12-science - Chemistry
Asked by kaushikmisty07 | 31 Dec, 2023, 11:42: AM
CBSE 12-science - Chemistry
Asked by kamlesh.kumar.malee | 20 Dec, 2023, 06:59: AM
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM








