CBSE Class 12-science Answered
HI SIR GOOD MORNING....SIR I HAVE A DOUBT IN ELEVATION OF BOILING POINT.....
Asked by sinkudad9 | 05 Oct, 2018, 07:57: AM
BOILING POINT ELEVATION:
- Vapour pressure of the solvent decreases in the presence of a non-volatile solute.
- Variation of the vapour pressure of the pure solvent and solution depends upon the temperature.
- For example, the vapour pressure of an aqueous solution of sucrose is less than 1.013 bar at 373.15 K.
In order to make this solution boil, its vapour pressure must be increased to 1.013 bar by raising the temperature above the boiling temperature of the pure solvent (water).
Thus, the boiling point of a solution is always higher than that of the boiling point of the pure solvent in which the solution is prepared.
This concept is known as Elevation of Boiling Point.
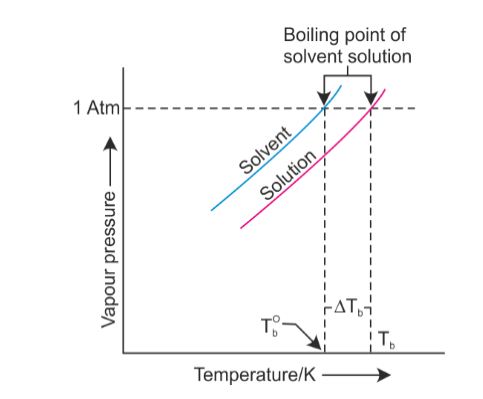
- The elevation of the boiling point depends on the number of solute molecules rather than their nature hence, it is a colligative property.
- Let Tb0 be the boiling point of the pure solvent and Tb is the boiling point of the solution.
The increase in the boiling point ΔTb = Tb - Tb0 is known as elevation of boiling point.
Answered by Varsha | 05 Oct, 2018, 11:43: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM
CBSE 12-science - Chemistry
Asked by vekariyaparth61 | 16 May, 2022, 04:33: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:27: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:20: PM
CBSE 12-science - Chemistry
Asked by sshashu993 | 25 Jul, 2020, 08:02: AM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 05:52: PM
CBSE 12-science - Chemistry
Asked by sharmasherryal | 25 May, 2020, 09:54: AM
CBSE 12-science - Chemistry
Asked by panthpreet0221 | 06 May, 2020, 10:41: AM