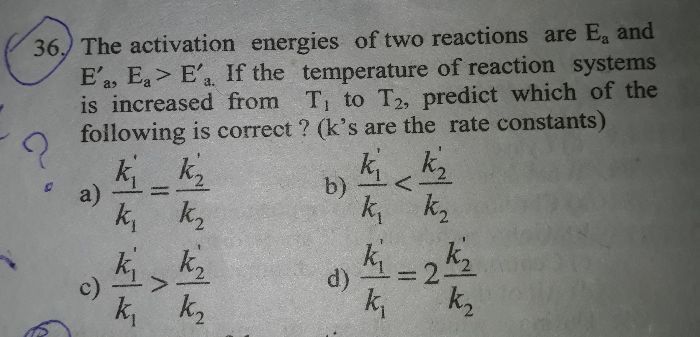CBSE Class 12-science Answered
effect of cone temperature of the rate of b/w sodium thiosulphur and hydrochloric acid.
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
The chemical reaction of sodium thiosulphate (Na2S2O3) with hydrochloric acid (HCl) produces a translucent colloidal sulphur solution.
The chemical equation for this reaction is as follows:
The chemical equation for this reaction is as follows:
Na2S2O3(aq) + 2HCl (aq) → 2NaCl (aq) + H2O(l) + SO2(g) + S(s)
The ionic equation for this reaction is:
S2O3-2(aq) + 2H+(aq) → H2O (l) + SO2(g) + S(s)
The rate of sulphur precipitation increases as the system's concentration or temperature rises.
This is due to the fact that increasing the concentration or temperature increases the kinetic energy of the reacting species, resulting in a greater number of successful collisions and hence a faster reaction rate.
The rate of chemical reaction is directly related to its temperature.
The reaction rate increases as the temperature rises.
The kinetic energy of the molecules increases as the temperature rises.
The pace of reaction is typically doubled for every 10-degree increase in temperature.
As a result, the rate of reaction between hydrochloric acid and sodium thiosulphate increases as temperature rises.
Answered by | 01 Dec, 2023, 11:41: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM
CBSE 12-science - Chemistry
Asked by amritha2960 | 13 May, 2020, 08:26: AM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 02 May, 2020, 09:20: AM
CBSE 12-science - Chemistry
Asked by leelakrishnapallapotu143 | 29 Mar, 2020, 07:27: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 30 Jan, 2020, 03:29: PM